RBQM And ML: Perfect Partners For More Efficient Clinical Trials
Artificial intelligence (AI) and risk-based quality management (RBQM) are growing trends in pharma – and the two go together like strawberries and cream. For decades, clinical trials have become increasingly expensive, time-consuming, and complex, leading sponsors to seek ways to streamline the process safely. Enter RBQM, which enables researchers to focus on what matters most […]
Twin Peaks: 10 Years Of CluePoints And Risk-Based Monitoring

It’s been ten years since CluePoints set out on a journey to let clinical trial data speak for itself – and what a journey it has been. For the first five years, we wondered if we would ever find success, and for the last five years, we have been wondering just how successful we could […]
The Inaugural ACDM AI & ML Conference Promises To Move The Dial
CluePoints is excited to participate in a brand-new annual conference designed to help the research sector level up its artificial intelligence (AI) and machine learning (ML) use. AI and ML have the potential to revolutionize clinical trial management, but not every company is ready to embrace the change. While some are already implementing solutions and […]
Natural Language Processing Improves Risk Signal Documentation In Clinical Trials
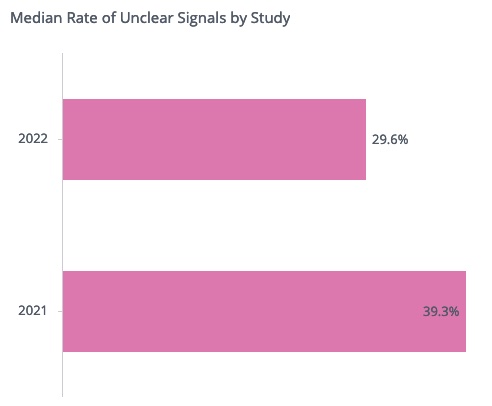
Authors: Sylviane de Viron, Data and Knowledge Manager Nicolas Huet, Machine Learning Manager Central monitoring helps sponsors proactively identify quality issues in clinical trials. To meet regulatory requirements, support continuous improvement, and enable further optimization of issue detection through machine learning, it is imperative to document the follow-up of central monitoring findings from initial detection […]
The Best Of Both: The Key Reasons To Outsource RBQM Tech But Keep Data Control

You can outsource the work, but you can’t outsource the responsibility – so should you outsource Risk-Based Quality Management (RBQM)? It’s a question pharma companies are increasingly asking themselves as the industry continues to adopt this new efficient way of working. Here, Rick Ward, Senior Vice President at CluePoints, talks us through the key considerations […]
10 Reasons Why CROs Leverage CluePoints For RBQM
Written by Andrea McSweeney, Vice President of Customer Success – Channel, CluePoints Contract research organizations (CROs) are uniquely positioned to partner with sponsors on risk assessment and monitoring activities—but they must have the systems in place to ensure subject safety and data quality across their study portfolios. CluePoints can offer the tools to aggregate data […]
CluePoints To Share Case Study On Technology ROI At The SCDM 2022 EMEA Leadership Forum & Conference
Decentralized clinical trials (DCTs) have become a buzzphrase in recent years, with commentators lining up to extol the benefits of this new way of working. Practical advice on how to adopt and implement the model, however, has been thinner on the ground. Organizations may still be wary of their return on investment (ROI) when it […]
Join Us At DIA 2022 As We Demystify The De-Risking Of DCTs
The world of decentralized clinical trials (DCTs) and risk-based quality management is evolving rapidly – but CluePoints and our partners are here to help you stay abreast of the latest developments. John Hall, the company’s Senior Vice President for EU and APAC, will lead a session on de-risking DCTs at this year’s Drug Information Association […]
10 Ways The Pandemic Changed CluePoints

AUTHOR: PATRICK HUGHES, CO-FOUNDER & CHIEF COMMERCIAL OFFICER, CLUEPOINTS As CluePoints celebrates ten years as the leading provider of Risk-Based Study Execution (RBx) and Risk-Based Quality Management (RBQM) software for clinical trials, co-founder Patrick Hughes looks back on the changes the company has undergone in that time – as well as takes a broader look at the […]
Does Central Monitoring Lead To Higher Quality? An Analysis Of KRI Outcomes

AUTHOR: SYLVIANE DE VIRON, MANAGER, DATA & KNOWLEDGE, CLUEPOINTS AND WILLIAM STEIJN, RESEARCH DATA ANALYST, CLUEPOINTS Central Monitoring is a key component of Risk-Based Quality Management (RBQM). Many organizations have now implemented it to enable and support pro-active quality oversight of clinical research. Central monitoring aims to detect emerging quality issues pro-actively during a clinical […]

