Risk-Based Quality Management (RBQM) Solutions
Leverage cutting-edge advancements in statistics and Machine Learning (ML) with AI-powered clinical trial solutions that go beyond traditional data analysis. By adhering to FDA and ICH guidelines and regulations, we accelerate result delivery to research teams, facilitating prompt issue resolution. Our RBQM technology identifies data outliers and anomalies in real time, offering researchers a better way to identify and manage clinical trial risks.
USERS
DE-RISKED
ISSUES DETECTED
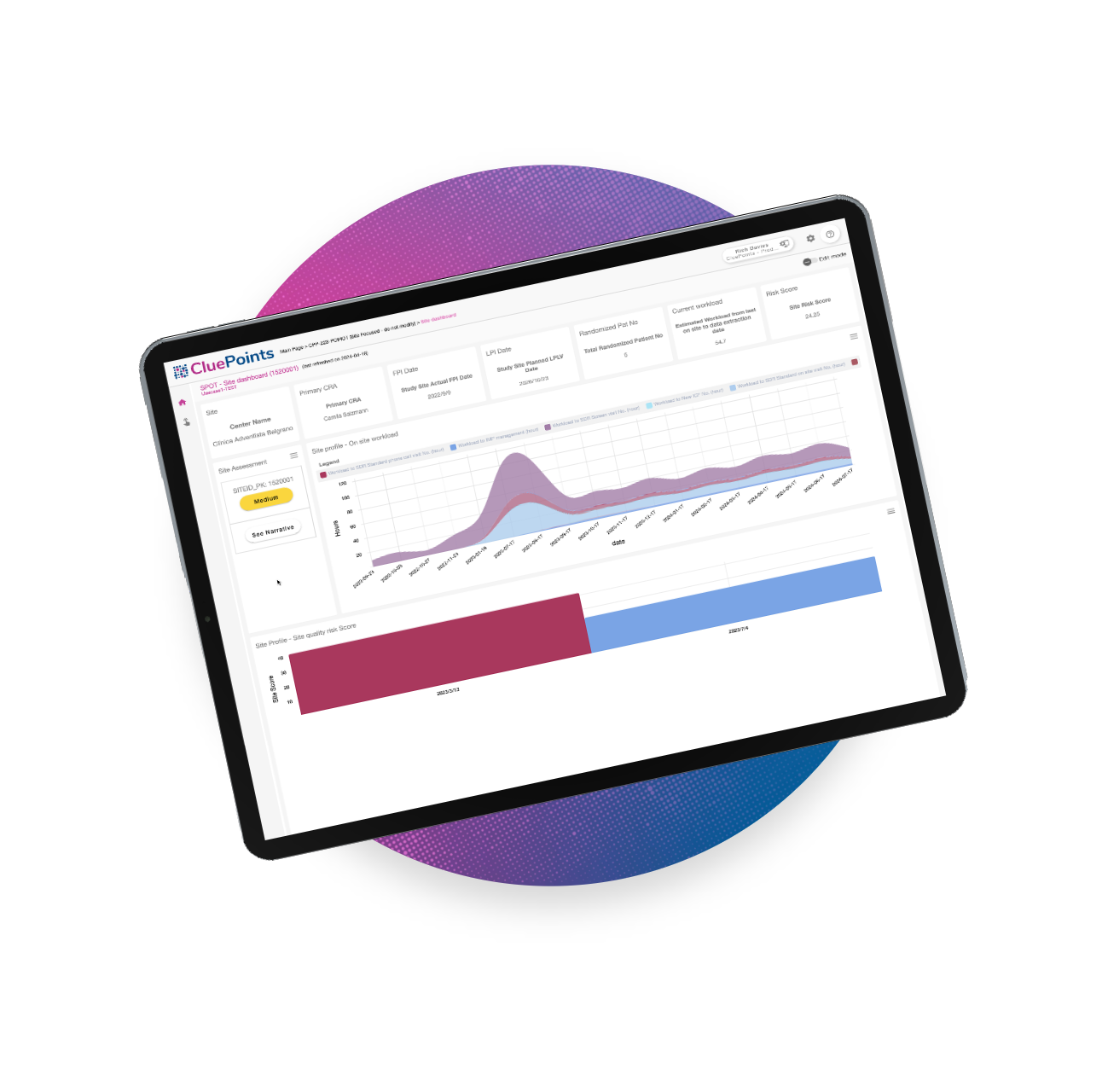
Proactive Risk Detection & Documentation
Leverage cutting-edge advancements in statistics and Machine Learning (ML) with AI-powered clinical trial solutions that go beyond traditional data analysis. By adhering to FDA and ICH guidelines and regulations, we accelerate result delivery to research teams, facilitating prompt issue resolution. Our RBQM technology identifies data outliers and anomalies in real time, offering researchers a better way to identify and manage clinical trial risks.
Key Risk Indicators (KRIs)
Rank clinical research sites based on performance and tolerance metrics, uncovering ‘at-risk’ locations in need of proactive corrective action.
Quality Tolerance Limits (QTLs)
Identify systematic issues, monitor key metrics, and document deviations from pre-defined QTLs to ensure accountability and compliance throughout clinical trials.
Central Statistical Monitoring
Test all collected clinical research data, including both clinical and operational, exposing atypical data patterns that indicate operational challenges.
Duplicate Patients
Generate a priority listing of patient pairs by specifying configurable variables likely to indicate duplicates and review based on side-by-side scoring and evaluations.
Patient Profiles
Flag atypical patient patterns, prioritize investigations into anomalies within patient data sets, and target those of utmost importance.
Business Intelligence (BI)
Explore clinical and operational data from various perspectives and domains in customizable formats with multiple data widgets.
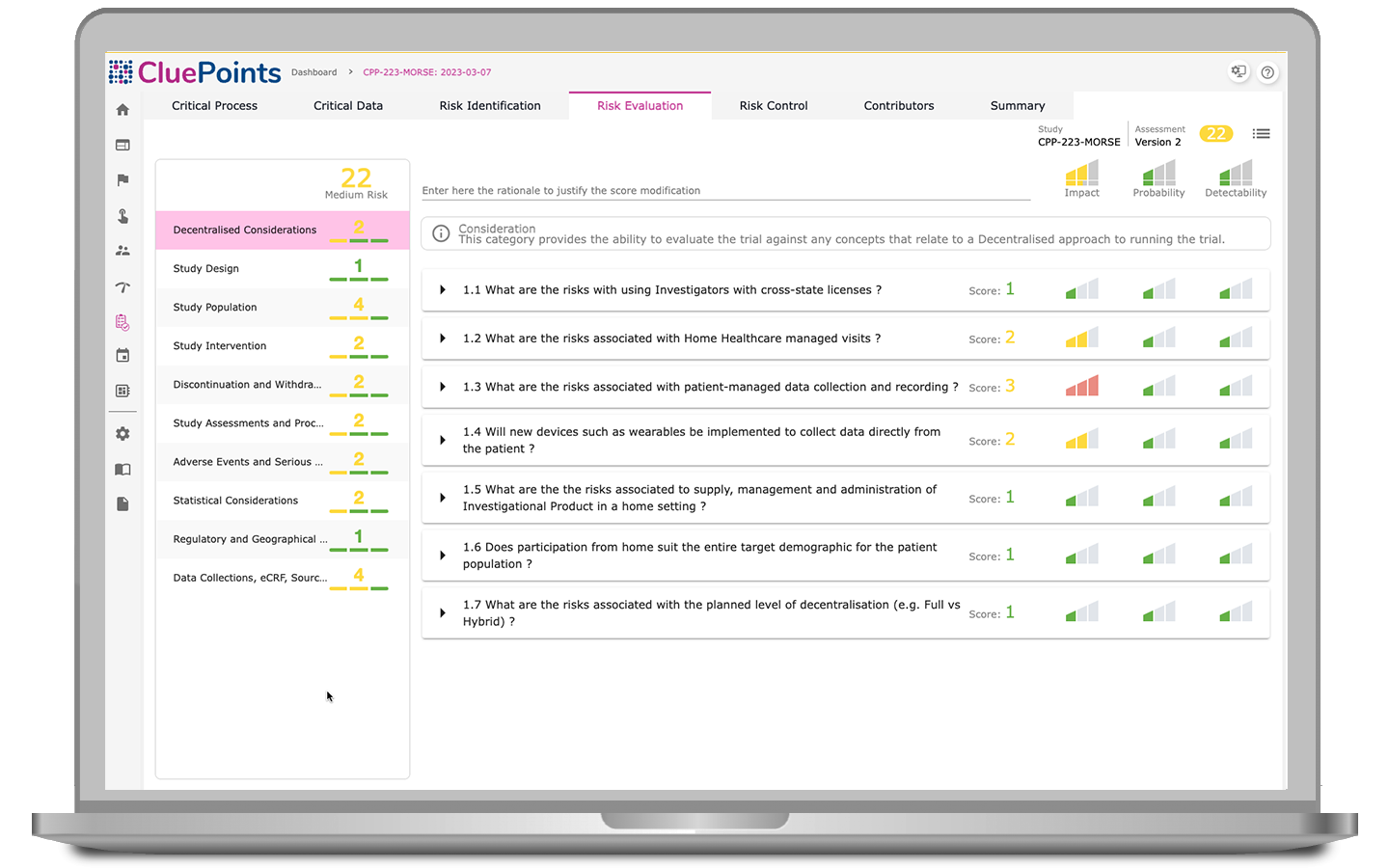
Risk Assessment & Mitigation
Determine vulnerable clinical trial areas, define risk controls, and monitor capabilities to detect evolving threat levels during study execution.
Signal & Action Tracker
Assess identified issues, annotate commentary throughout signal processing, deploy automatic auditing, and leverage extensive exporting capabilities.
Data Analytics & Risk Detection
- Key Risk Indicators (KRIs)
- Quality Tolerance Limits (QTLs)
- Central Statistical Monitoring
- Duplicate Patients
- Patient Profiles
- Business Intelligence (BI)
Clinical Trial Management & Documentation
- Risk Assessment & Mitigation
- Signal & Action Tracker
Clinical Trial Expertise & Client Services
- Central Monitoring
- RBM/RBx Implementation
- Site Inspection Readiness
- Mergers & Acquisitions

The Ultimate Guide to Risk-Based Quality Management (RBQM)
RBQM Solutions for Every Clinical Trial
CluePoints provides our community with smarter ways to run clinical trials. By leveraging the power of AI using advanced statistics and machine learning, CluePoints’ customers and partners can harness its proven, best-in-class platform of solutions to develop new therapies to improve the lives of people worldwide.
Contract Research Organizations (CROs)
Pharmaceuticals
Medical Devices
Academic Community
The Bold Future of RBQM for CROs
In a world where complacency is the enemy of progress, Contract Research Organizations (CROs) stand at a crossroads. Traditional approaches have their merits, but they’re no match for the transformative power of risk-based quality management (RBQM). It’s time to be bold, challenge the status quo, and redefine the future of CROs.