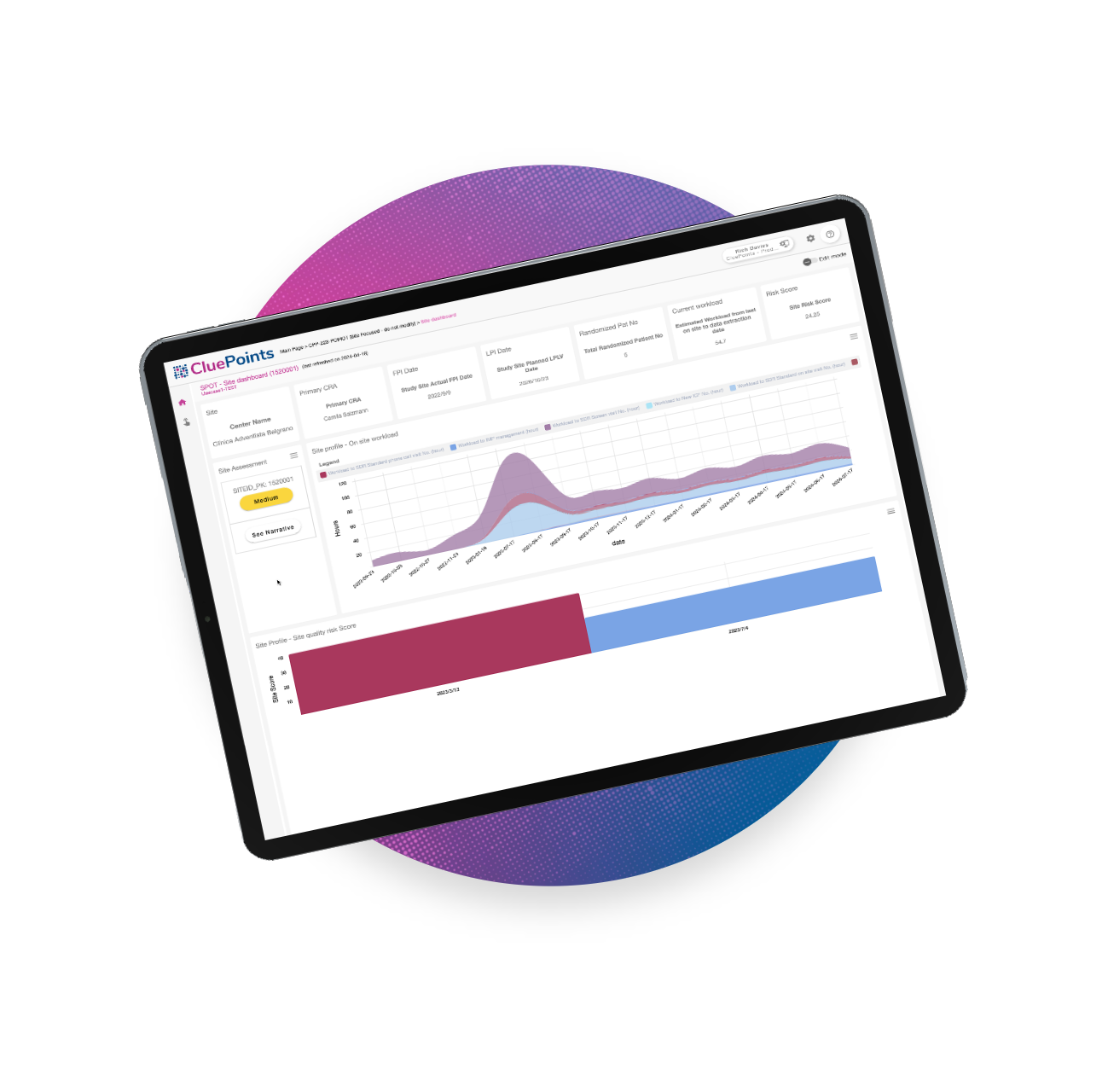
Site Profile & Oversight Tool (SPOT)
SPOT enables adaptive site monitoring so clinical research teams can swiftly pinpoint anomalies and translate insights into actionable strategies. Sponsors and CROs can improve their ability to evaluate the performance of clinical trial sites and adjust site visitation plans more effectively and efficiently. These adjustments more accurately consider research risk and resource workload.
USERS
DE-RISKED
ISSUES DETECTED
Efficient Adaptive Site Monitoring
Alleviate the time-consuming aspect of clinical research site monitoring activities and leverage technology that surpasses human capabilities.
Data-Driven Research Site Scoring
Implement a configurable calculation that incorporates central data review risk, onsite workload, and other relevant factors.
User-Triggered Actions
Initiate actions based on identified insights, enabling timely responses and proactive management.
Automated Actions
Automatically detect and respond to key triggers to swiftly identify actionable insights without user intervention.
Clinical Trial Risk Mitigation
Reduce exposure to clinical trial risk and avoid potentially costly issues, improving overall study outcomes and success.
Insightful Data Visualization
Consolidate disparate data sources into a single, summarized view to inform decisions and take action quickly.
Simple Data Integration
Centralize diverse data types to enhance site management strategies, data integrity, and decision-making.
Ensure Research Accountability
Equip study leadership with visibility into the actions and reviews of site managers, site relationship managers, and CRAs.
Accessibility for All
Implement role-based security measures so that users have the appropriate access to data at the right time, fostering a secure, efficient environment.
Flexible & Tech-Agnostic Platform
Integrate SPOT with existing systems, minimizing implementation effort and maximizing the value of existing tools.
Effective Site Visit Planning
Our solution offers dynamic adjustments for research site visits, taking into account anticipated site workload and central data review risk. With this capability, users can optimize monitoring schedules to avoid unnecessary delays, ensure resources are allocated effectively, and mitigate risks promptly.
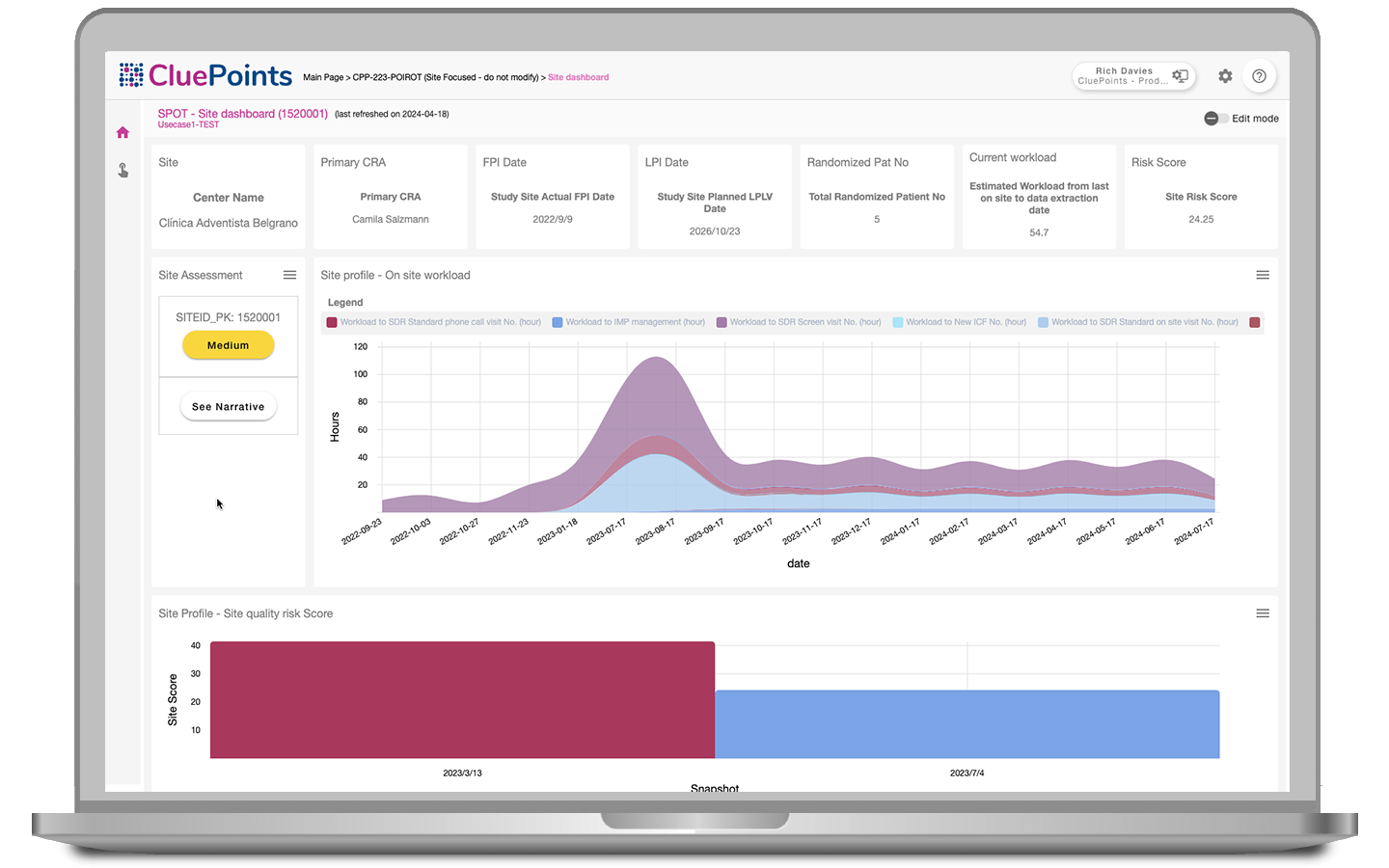
Streamline Site Performance Evaluation
Streamline site performance evaluations and save time with SPOT. CluePoints eliminates labor-intensive manual assessments, enabling study managers and CRAs to evaluate performance and take prompt corrective actions.

Productive Site Recruitment
By harnessing valuable insights on site performance, users can pinpoint highly successful studies and replicate effective site selection and recruitment approaches, maximizing study efficiency and success rates through data-driven recruitment strategies.

Targeted SDV & SDR Strategies
Customize your approach to source data verification (SDV) and source data review (SDR) with precision. Tailor fit-for-purpose verification and review strategies to meet the unique needs and risks of each study, eliminating unnecessary efforts and optimizing resource allocation.

CRA Performance Evaluation
Gain insight into CRA performance utilizing real-time data on site interactions to assess CRA effectiveness across studies. SPOT empowers users to make informed decisions regarding CRA assignments and training, ensuring optimal performance and success across clinical trials.

Tailored Implementation Support
From tailored assessment workshops to meticulous pilot development and ongoing support, we guide you through every step of implementation. Seamlessly transition to SPOT with expert knowledge transfer training and validation support. Maximize efficiency and effectiveness with customized SPOT dashboards, action templates, and data transformation capabilities. Collaborate with our dedicated team and leverage resources to ensure smooth implementation and ongoing utilization.

SPOT for Every Clinical Trial
CluePoints provides Sponsors and CROs with smarter ways to evaluate the performance of clinical trial sites and identify anomalies. By leveraging the power of AI using advanced statistics and machine learning, CluePoints’ customers and partners can harness its proven, best-in-class platform of solutions to develop new therapies to improve the lives of people worldwide.
Contract Research Organizations (CROs)
Pharmaceuticals
Medical Devices
Academic Community
Transforming Site Monitoring Practices with Adaptive Intelligence
Site monitoring is one of the most expensive components of clinical trials; on-site monitoring alone accounts for around 25 to 30% of the total trial expenses.1 A substantial portion of the clinical trial timeline involves inefficient Source Data Verification (SDV) and Source Data Review (SDR) activities, which can also consume a significant part of a monitoring budget and overall trial duration. The industry needs more efficient site monitoring processes to streamline clinical trials, reduce both time and costs, and ensure better accuracy.