International Women’s Day: Catherine Ditzler
Here’s to strong women. May we know them, may we raise them, may we be them.’ In a new series of blogs to mark International Women’s Day, VPs from across CluePoints share their experiences, challenges, and advice for anyone wanting to pursue success. Catherine Ditzler, CluePoints VP of Marketing, believes everyone should be inspired and supported […]
Leveraging RBQM In Ways You Never Envisioned
Risk-Based Quality Management (RBQM) is fast becoming a staple in clinical research, and the community is still learning new ways in which the approach can add value to drug development and sponsor organizations. From monitoring sustainability metrics to enabling proactive inspection readiness, the model, which was mandated by the International Council for Harmonization (ICH)’s guideline […]
Harnessing Risk-Based Quality Management And Deep Learning To Improve Trial Knowledge And Drive Better Outcomes – Your Questions Answered
Risk-Based Quality Management (RBQM) adoption continues to rise, ensuring effective oversight of disparate data sources within a clinical trial. Integrating deep learning capabilities has only enhanced RBQM’s potential to improve trial knowledge and drive better outcomes for sponsors and patients. In our latest blog Steve Young, Chief Scientific Officer at CluePoints, answers your questions on […]
The Importance Of Risk Assessment As A Foundation Of RBQM
Author: Rod Rahimi, Solution Expert at CluePoints. Risk assessment is the foundation of Risk Based Quality Management (RBQM). The adoption of statistics to assist with quality oversight is well documented, leading to the evolution of RBQM from a ‘nice-to-have’ into a ‘must-have’ in clinical trials. However, there can be a temptation to jump straight into […]
10 Reasons People Like Working At CluePoints
From the friendly, supportive culture to making a difference in people’s lives, there are a million and one reasons why people love working at CluePoints. So when we asked our staff what they liked most about their job, narrowing the answers down to ten was a challenge. But, to mark a decade since CluePoints first […]
RBQM And ML: Perfect Partners For More Efficient Clinical Trials
Artificial intelligence (AI) and risk-based quality management (RBQM) are growing trends in pharma – and the two go together like strawberries and cream. For decades, clinical trials have become increasingly expensive, time-consuming, and complex, leading sponsors to seek ways to streamline the process safely. Enter RBQM, which enables researchers to focus on what matters most […]
Twin Peaks: 10 Years Of CluePoints And Risk-Based Monitoring

It’s been ten years since CluePoints set out on a journey to let clinical trial data speak for itself – and what a journey it has been. For the first five years, we wondered if we would ever find success, and for the last five years, we have been wondering just how successful we could […]
The Inaugural ACDM AI & ML Conference Promises To Move The Dial
CluePoints is excited to participate in a brand-new annual conference designed to help the research sector level up its artificial intelligence (AI) and machine learning (ML) use. AI and ML have the potential to revolutionize clinical trial management, but not every company is ready to embrace the change. While some are already implementing solutions and […]
Natural Language Processing Improves Risk Signal Documentation In Clinical Trials
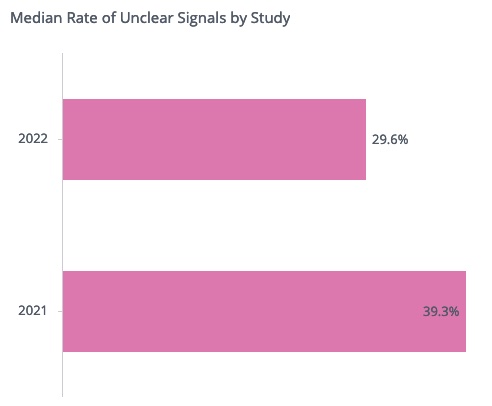
Authors: Sylviane de Viron, Data and Knowledge Manager Nicolas Huet, Machine Learning Manager Central monitoring helps sponsors proactively identify quality issues in clinical trials. To meet regulatory requirements, support continuous improvement, and enable further optimization of issue detection through machine learning, it is imperative to document the follow-up of central monitoring findings from initial detection […]
The Best Of Both: The Key Reasons To Outsource RBQM Tech But Keep Data Control

You can outsource the work, but you can’t outsource the responsibility – so should you outsource Risk-Based Quality Management (RBQM)? It’s a question pharma companies are increasingly asking themselves as the industry continues to adopt this new efficient way of working. Here, Rick Ward, Senior Vice President at CluePoints, talks us through the key considerations […]



