Efficient Medical & Safety Review (MSR) Solutions for Clinical Trials
CluePoints’ MSR tool centralizes and standardizes clinical data review processes, providing pre-made and customizable review dashboards for efficient query management throughout a clinical trial. This is especially valuable for Clinical Data Managers and Medical and Safety Reviewers for pharma companies and CROs, who rely on manual processes to analyze patient data and safety outcomes from EHRs, lab results, and other sources during clinical trials—methods prone to error and inefficiency.
USERS
DE-RISKED
ISSUES DETECTED
Medical and Safety Review (MSR) Tool’s Key Features
Improve pharmacovigilance and medical review efficiencies through streamlined workflow communication and AI-powered clinical trial management solutions.
Automated Record Change Monitoring
Identify changes in reviewed records, ensuring efficient evaluation of data points and high record quality.
Full History of Records
Track actions associated with a given record, including data point creation or updates, comments, queries, changes in assignee or status category, and more.
Frequent Data Refreshes
Receive frequent data updates to address emerging trends and potential clinical trial safety issues, ensuring proactive, informed decision-making and enhanced organizational communication.
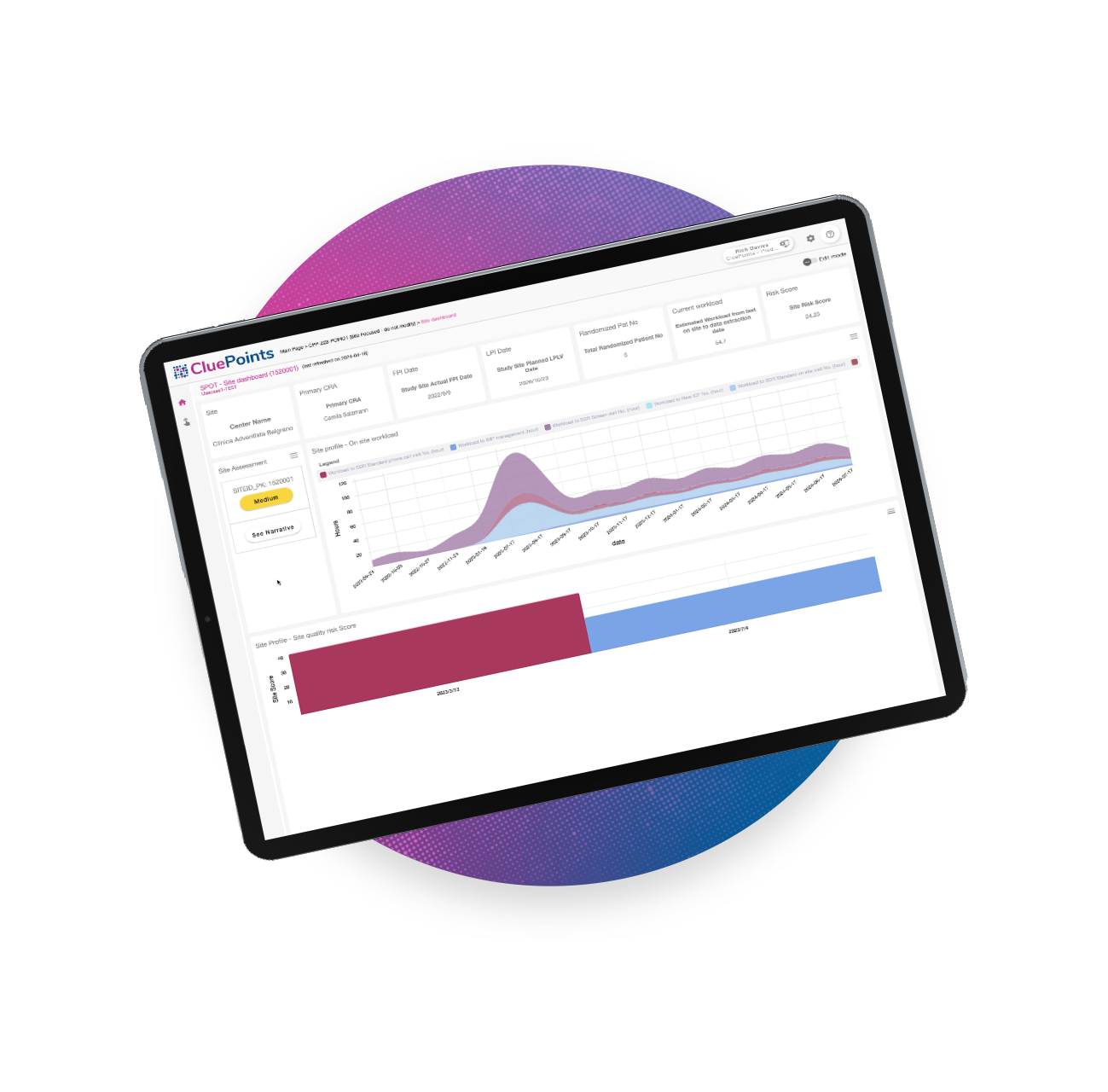
Customizable Dashboards
Empower users to create their own custom dashboards or choose from a library of existing ones, simplifying clinical data exploration and visualization preparation.
Automated Detection Rules
Process and flag records of interest using defined filter criteria to enhance the efficiency of clinical data review.
Centralized Query Integration
Raise EDC queries and ensure they’re all logged, updated, documented, and resolved within a single platform.
User-Friendly Review Workflow
Track individual record reviews with the ability to comment and assign records to other reviewers.
Interactive Dashboards
Filter, sort, and drill down into research study data to expertly visualize and interact with information, delivering insights at a glance
Streamlined Study Visualization
With a comprehensive visualization library and the ability to copy, reuse, and customize dashboards for specific study data requirements, MSR significantly reduces the time spent on clinical study preparation. Teams can quickly set up tailored visualizations, optimizing preparation time, efficiency, and internal alignment.
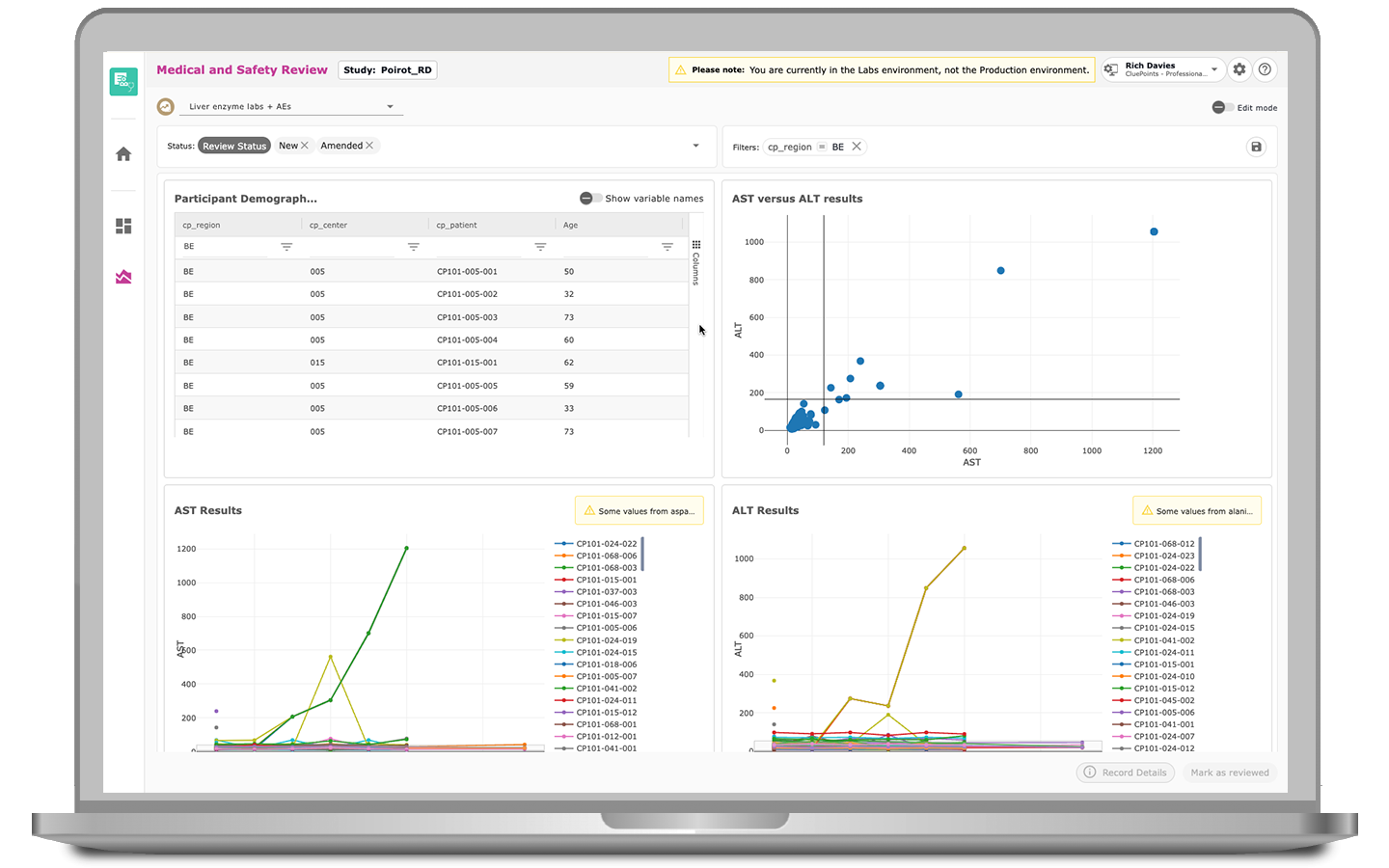
Fast Outlier Identification
MSR features predefined filters for rapid and accurate record identification, ensuring potential issues are flagged early in the process. As query management progresses, MSR offers clear visibility of current statuses, tracks changes, provides comprehensive EDC query feedback, and streamlines user assignments.

Consistent Review Process
MSR ensures that the medical or clinical trial safety review process is consistent regardless of the reviewer or what study they’re reviewing. By highlighting variable-level delta changes with calculations from the previous review, MSR simplifies tracking and minimizes variability, ensuring medical and safety review teams maintain high-quality reviews.

Seamless System Integration
MSR integrates research data review and query management processes, reducing the frequency of system swaps. It automatically communicates queries to the source system via API, ensuring an efficient workflow. Paired with CluePoints’ broader offerings, MSR and RBQM provide a comprehensive approach to clinical trial optimization.

MSR for Every Clinical Trial
CluePoints provides Sponsors and CROs with a smarter way to identify outlying values, track changes, and improve workflow communication. By leveraging the potential of AI using advanced statistics and ML, CluePoints is transforming a powerful, best-in-class platform of clinical research trial solutions into positive clinical development outcomes as our partners work to develop innovative therapies to improve the lives of people worldwide.
Contract Research Organizations (CROs)
Pharmaceuticals
Medical Devices
Academic Community
Why Data Accuracy Matters in Clinical Trials
Accurate data is the backbone of successful clinical trials. Every piece of patient data, from electronic health records (EHRs) to lab results, holds critical value for decision-making processes, influencing not only trial outcomes but also patient safety. Traditional, manual methods of medical and safety reviews often struggle to keep data fresh, consolidated, and error-free, and failing to do so can cause inefficiencies and potentially put patients at risk.