Clinical Trial Risk Detection & Research Analytics Solutions
Utilize AI-powered centralized statistical monitoring to help researchers uncover insights commonly missed by traditional data quality control methods. Ensure focused and productive data analysis on the most pertinent data points. With CluePoints’ advanced analytics, you can navigate clinical trials with greater efficiency and confidence.
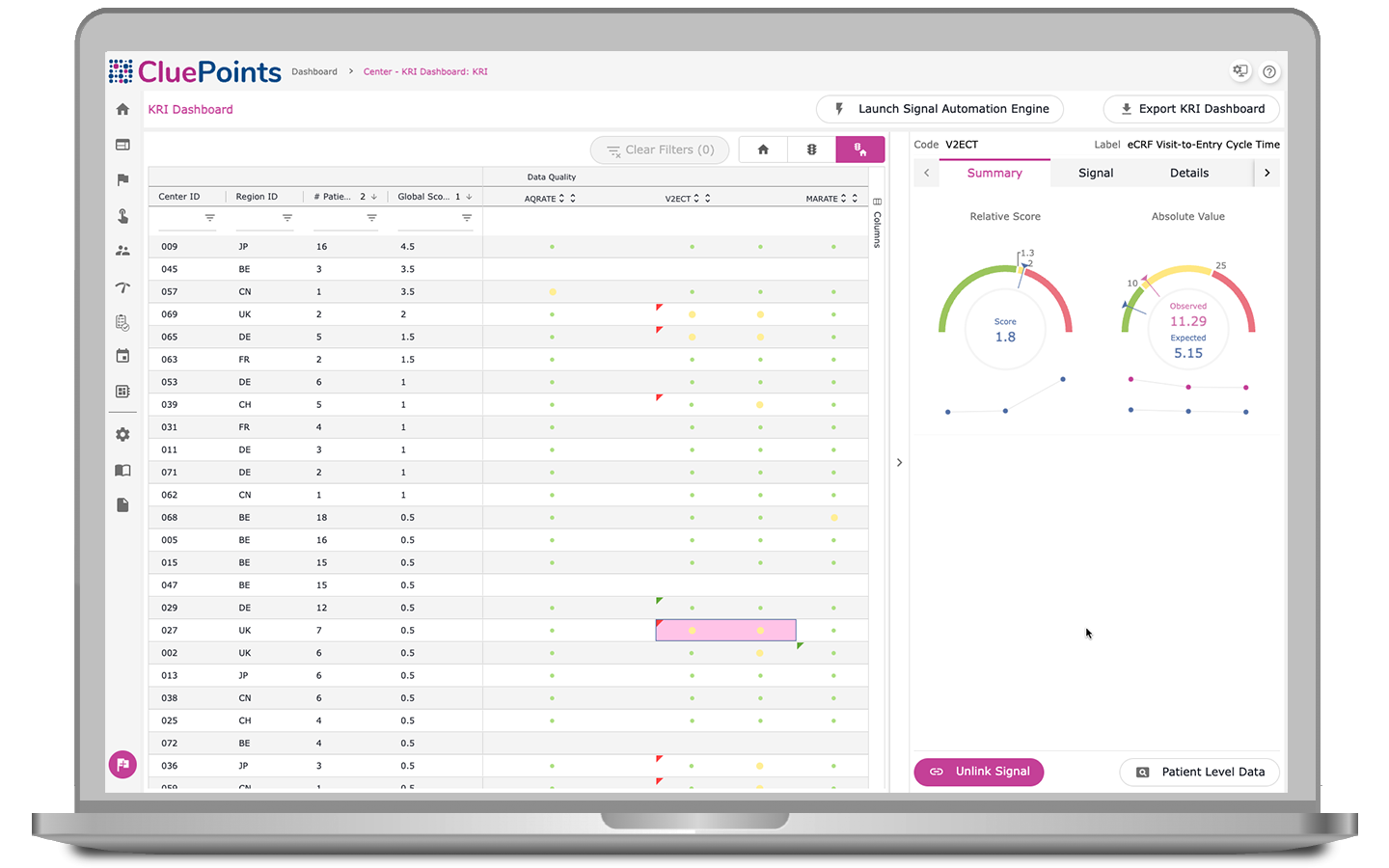
Key Risk Indicators (KRIs)
By harnessing statistical algorithms and subjective thresholds, our KRI dashboard ranks research sites based on performance metrics, spotlighting ‘at-risk’ clinical locations. Users can select from pre-defined KRIs or adapt indicators to clinical trial data, which yields scores that benchmark research site performance against peers and quality tolerance limits, pinpointing causation and ensuring proactive corrective actions through an integrated issue-tracking management system.
- Measure non-serious adverse events per patient visit for safety monitoring
- Track serious adverse events per patient visit for safety assessment
- Indicate missed patient assessments compared to expected
- Monitor deviations from study protocol per patient visit for adherence
- Measure patient visits conducted outside allowable schedules
- Calculate the average time from patient visit to eCRF entry
- Determine the rate of auto-queries per data point submitted
- Evaluate the average time from query generation to response
- Assess the proportion of screen-failed patients to the total screened
- Measure the rate of early-terminated patients per visit
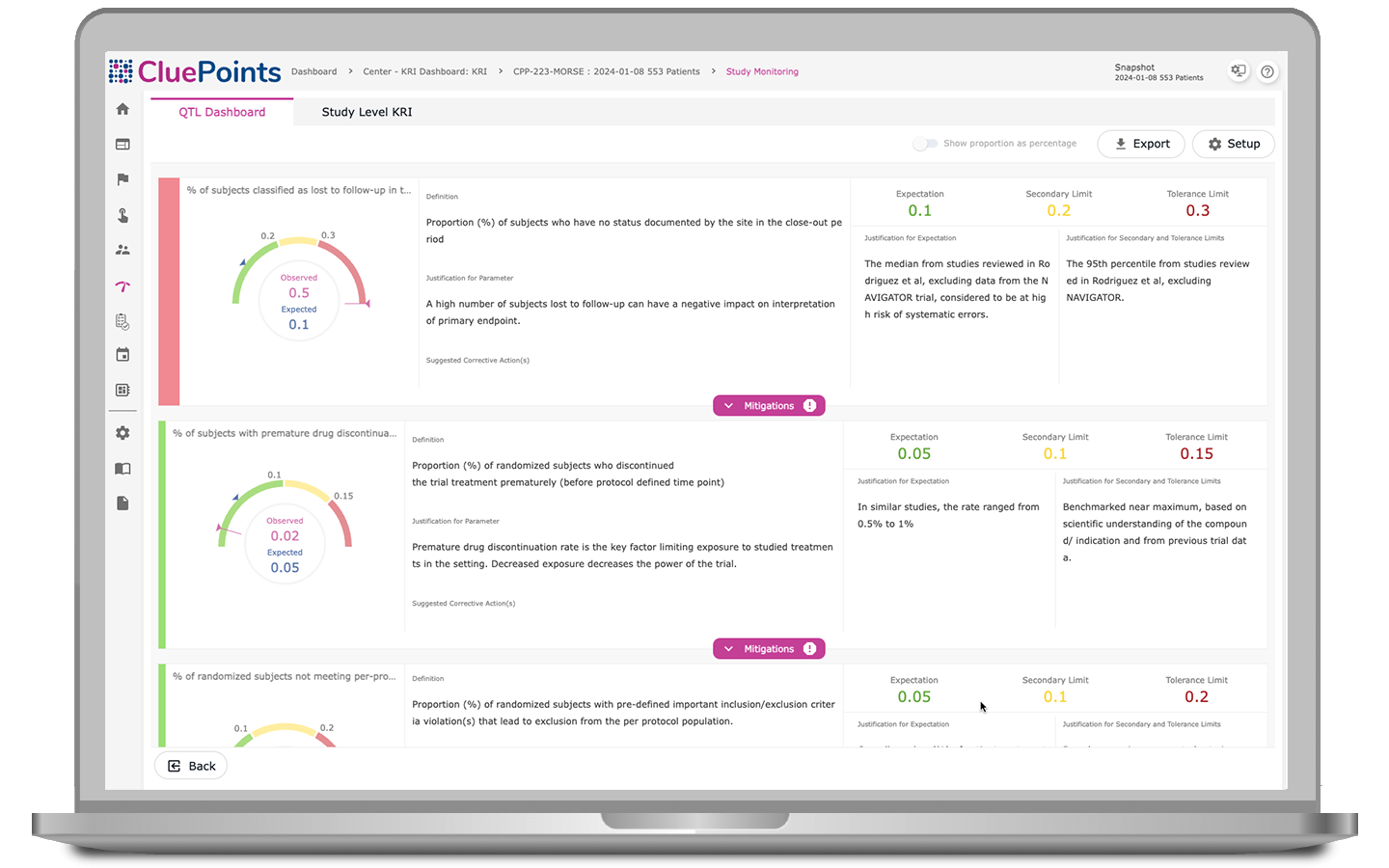
Quality Tolerance Limits (QTLs)
Our QTL module automatically identifies systematic issues, monitors key metrics, and documents deviations from pre-defined QTLs. Our platform automatically uncovers threats to trial validity and patient safety, enabling early issue detection for prompt corrective action. With comprehensive documentation of QTLs and breaches, including justification and evidence, all in one accessible platform, teams can ensure accountability and compliance throughout the clinical trial process.
- Monitor the rate of patients discontinuing study drug treatment prematurely
- Monitor the rate of enrolled patients lost to follow-up
- Monitor the rate of patients terminating participation before reaching an endpoint milestone
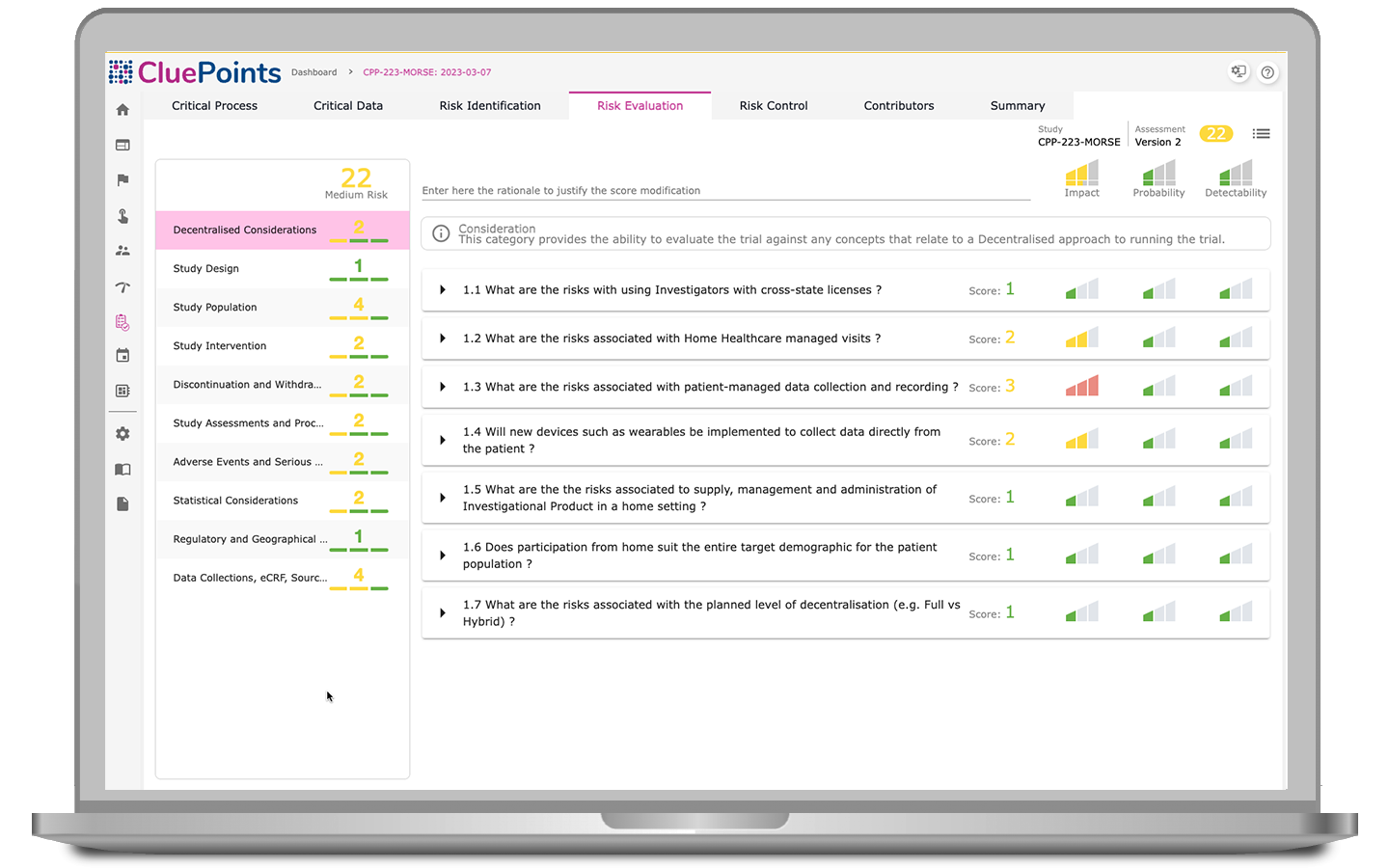
Central Statistical Monitoring
- Identify any instances of fraudulent activity or inaccuracies associated with patient information
- Identify errors in data recording, including improper rounding or digit manipulation
- Identify widespread errors stemming from equipment malfunctions or other systemic issues
- Identify any biases or deficiencies in the training of raters or assessors
- Identify data timeliness issues, such as implausible data accrual rates
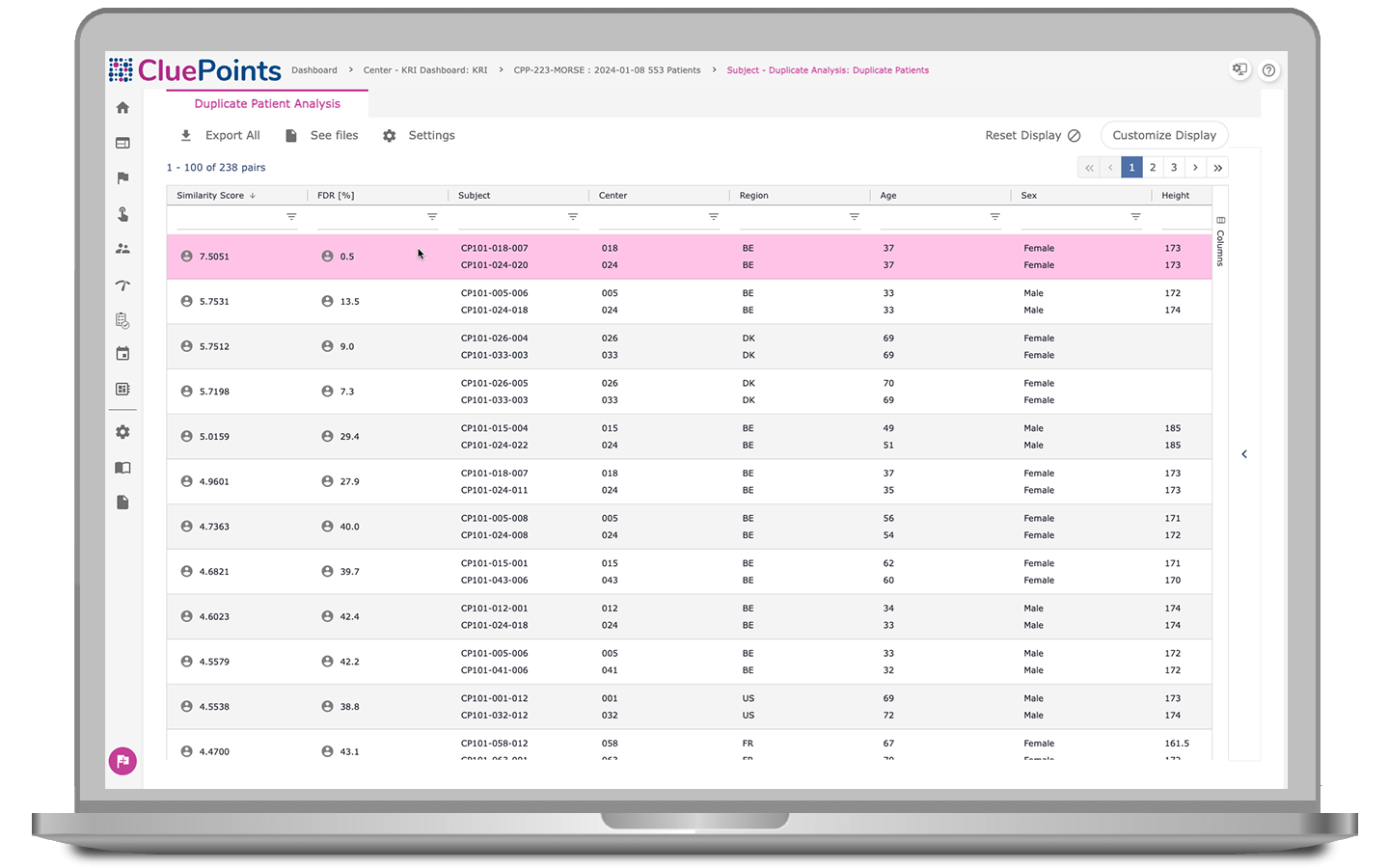
Duplicate Patients
- Address challenges posed by clinical site proximity, particularly in regions like North America where states such as California, Florida, Pennsylvania, and New York serve as hubs for research sites
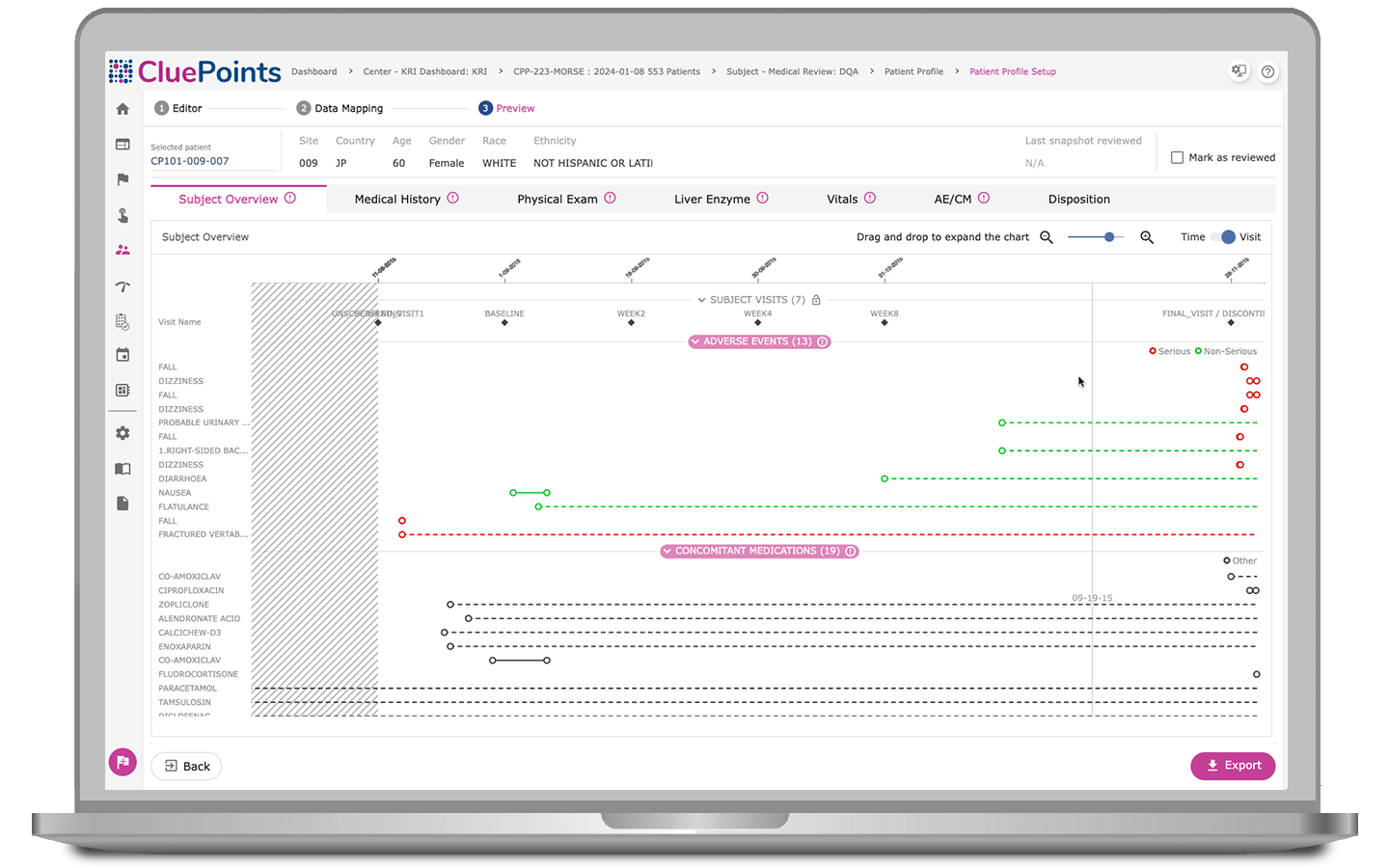
Patient Profiles
- Characterize risk signals quickly and effectively, enabling centralized medical and safety reviews
- Assess chronological views of patients’ visits, investigational product exposure, adverse events, concomitant medications, and other pertinent patient information
Business Intelligence (BI)
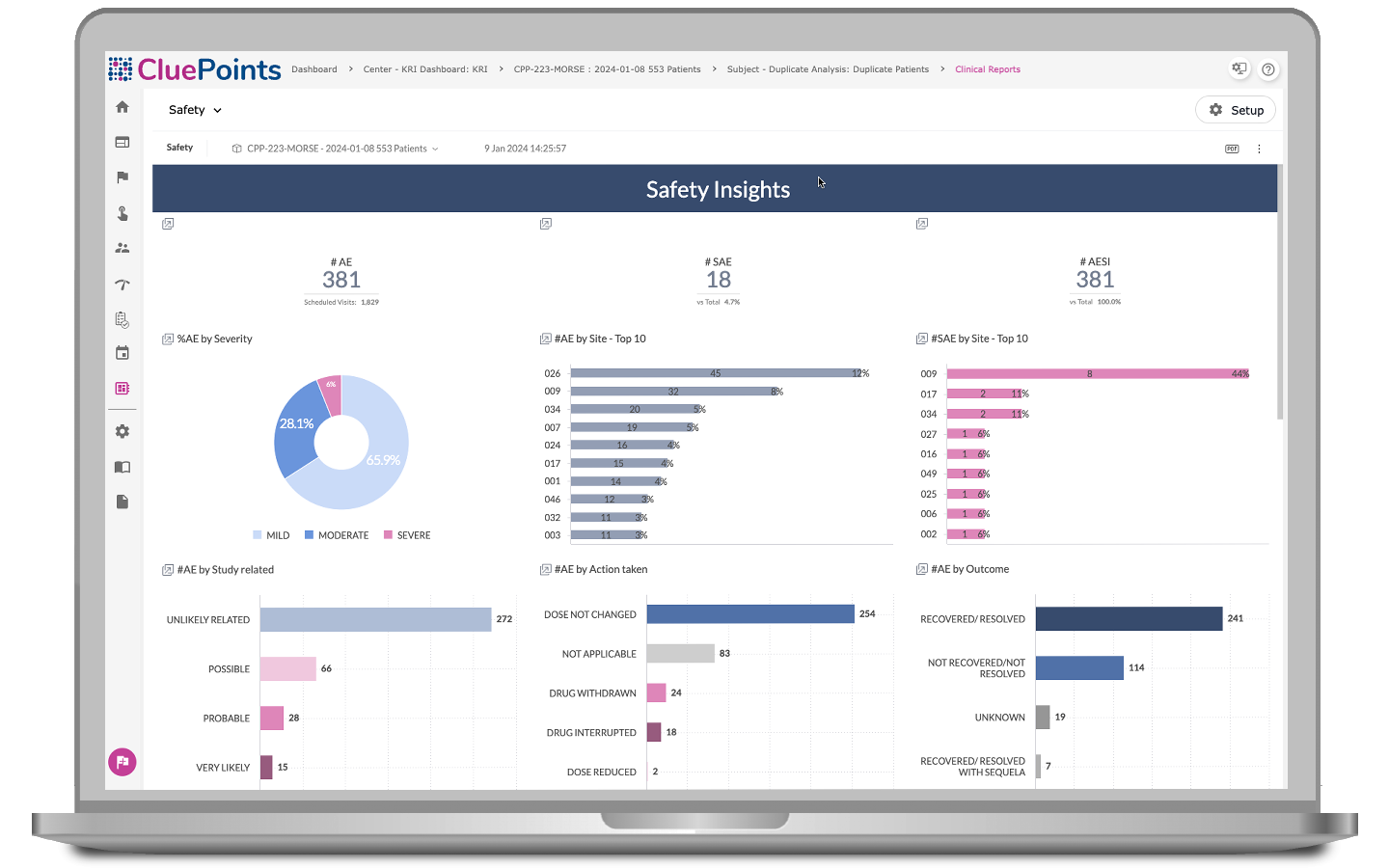
BEYOND is an advanced data visualization solution that goes beyond traditional statistical analysis, enabling users to explore clinical and operational data from various perspectives and in customizable formats. With multiple data widgets and combined data from different domains, users can summarize and aggregate data effectively and focus on specific aspects of trial operations, facilitating the identification of emerging risks and operational challenges.
- Focus on safety-related data, allowing for top-down analysis of adverse event distribution
- Examine outliers, identify associated adverse events and medications, and make comparisons over time
- Integrate forecast data for further insights into predicted versus actual enrollment and screen failure rates
- Extend the view to compute averages across sites for a thorough assessment of operational metrics
On-Demand Demos
Dive into the full potential of CluePoints’ AI-powered RBQM solutions right from the comfort of your screen.

