RBQM Doesn’t Have To Be Complicated, Risky Or Expensive: Q&A
On Wednesday, June 24, 2020, CluePoints delivered a webinar to demonstrate how using a combination of powerful analytics and comprehensive data visualization can be employed to ensure that no stone is left unturned in determining what issues are evident in studies and how to course-correct for a successful submission. If you missed the webinar, you […]
Beyond COVID-19: The New Normal In Risk-Based Monitoring (RBM) And Quality Management (RBQM)
Join our next webinar to learn first-hand how the CluePoints and Parexel partnership is driving best-in-class Risk-Based Quality Management Strategies to support clinical development. This session will focus on how early risk detection can be leveraged to detect issues across your trial and ensure operational excellence. As the COVID-19 pandemic continues, actions need to be prioritized to […]
Early Risk Detection During The COVID-19 Crisis – Your Questions Answered
On Tuesday, March 31, 2020, CluePoints delivered a webinar centred around the critical importance of early risk detection. Our presenter also shared insights on how to navigate risk management and risk assessment during the COVID-19 crisis. If you missed the webinar, you can watch it on-demand. Watching the webinar before digesting the answers shared in this document […]
Complimentary COVID-19 Risk Assessment Package Available To All At No Cost
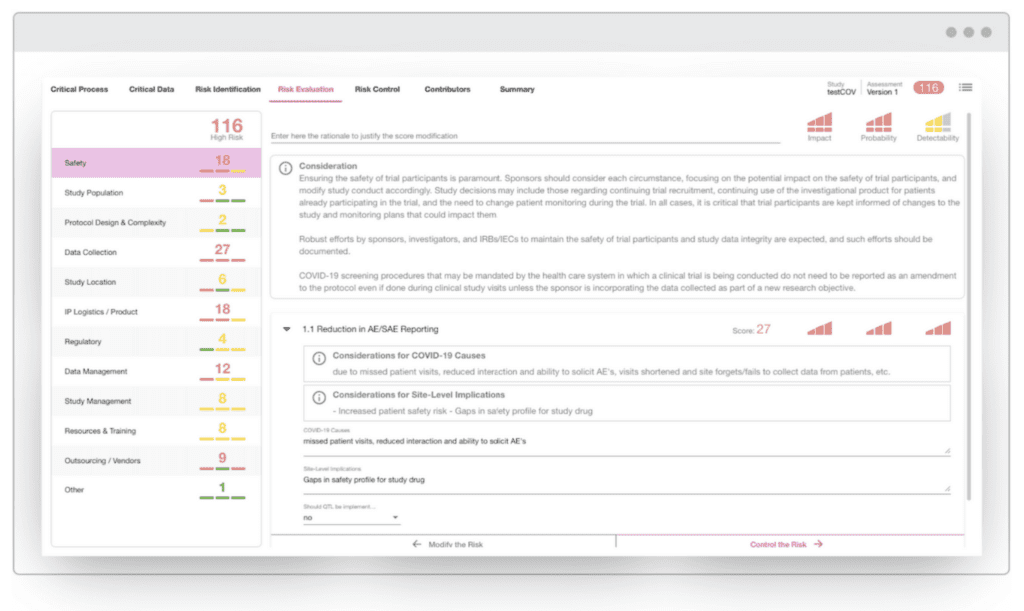
COVID-19 Risk Planning As advised in the coronavirus guidances recently issued by FDA, EMA, and MHRA, it is important to perform a fresh risk assessment for each study to identify and mitigate risks pertinent to the COVID-19 crisis. Your organization is likely already aware of key challenges that will be common to most if not […]
Implementing A Risk-Based Quality Management Strategy – The Ultimate Guide
Every organization faces unique challenges when it comes to implementing a Risk-Based Quality Management strategy. There’s no ‘one-size-fits-all’ approach that will ensure success, as each organization has specific influencing factors such as therapeutic areas, existing processes, and various technologies. Our latest guide, produced by our Chief Scientific Officer, Steve Young, takes a look at considerations for ensuring not […]
Transitioning From A Risk-Based Monitoring Approach To Risk-Based Quality Management
This content was originally posted on Applied Clinical Trials on March 18, 2019 One of the most significant positive changes we have seen recently is the endorsement of ICH E6 (R2) which insists that Sponsors and CROs adopt a risk-based approach to study execution. This paradigm shift in the industry ensures that Sponsors undertake risk assessment during […]
ICH E6 R2 – The Clinical R&D Industry’s Response
Looking for the most up-to-date perspective on ICH GCP? Explore our deep dive into the latest ICH E6(R3) Guidance and what it means for modern RBQM practices, including key changes, timelines, and practical implications for Sponsors and CROs. Read it here. For decades now the complexity, cost, and duration of clinical development have been steadily […]
Beyond Risk-Based Monitoring: How Intelligent Analytics Are Being Used To Drive Value And Support ICH Compliance

The complexity and size of clinical trials has increased dramatically – in parallel with growing costs and mounting regulatory pressures. The introduction of the finalized International Council for Harmonization’s (ICH) addendum to the ICH E6 Guideline for Good Clinical Practice (ICH E6 R2) earlier this year means that organizations across the industry are currently reviewing the update […]
The Bigger Picture Of ICH E6 R2: Looking Beyond Compliance
Journal for Clinical Studies Steve Young, Chief Operations Officer, CluePoints The introduction of ICH E6 (R2) this past year has rendered the implementation of risk-based monitoring principles a matter of GCP compliance. While clinical research organizations across the industry are now finally compelled to study the new guidance in order to roll out a compliant RBM strategy, […]
ICH E6 R2: Talking Big Pharma’s Response To The Addendum
This article was originally produced for Clinical Informatics News The introduction and implementation of ICH E6 R2 has raised many questions surrounding operational risk-based approaches to clinical trial oversight. With the industry now in the process of getting to grips with more modernized practices for study conduct mandated by the guidance, this article addresses how sponsors are working […]

