TL;DR Summary: Here are key definitions from our blog, “From Risk-Based Monitoring (RBM) to Risk-Based Quality Management (RBQM).”
- Risk-Based Monitoring (RBM): A strategy that uses software, data, and analytics to prioritize oversight on the most critical trial data and processes. RBM improves data quality, enhances patient safety, and reduces costs by enabling sponsors to identify and correct issues in real time.
- Risk-Based Quality Management (RBQM): An evolution of RBM that applies risk-based thinking across the entire clinical trial lifecycle from design through close-out. Reinforced by ICH E6(R3), RBQM embeds proactive risk identification, control, and continuous review, making it a regulatory expectation for modern clinical development.
- Central Statistical Monitoring (CSM): A core component of RBQM that analyzes all clinical and operational data to detect anomalies and discrepancies that traditional methods might miss. CSM supports fraud detection, quality oversight, and ongoing risk mitigation through data surveillance, Key Risk Indicators (KRIs), and Quality Tolerance Limits (QTLs).
A review of FDA marketing submissions between 2000 and 2012 revealed that roughly one-third (32%) of all first-cycle review failures, 16% of submissions overall, were driven by quality issues. Why was this? While the pharmaceutical industry had enjoyed economic growth in the 1990s, drug makers started to face growing pressure at the turn of the century from multiple directions:
- Highly publicized safety issues with marketed drugs: A series of high-profile withdrawals and lawsuits—such as concerns over cardiovascular risks in certain blockbuster medications—sparked public mistrust and increased regulatory scrutiny. Safety failures damaged reputations, slowed approvals, and added costly post-marketing requirements.
- A slowing of innovation: Despite soaring investment in research, the pace of novel drug discovery diminished. Many new approvals were incremental improvements or “me-too” drugs, rather than breakthrough therapies. The “low-hanging fruit” of easy drug targets had largely been picked, leaving more complex diseases and biology to tackle.
- Patent expirations: Blockbuster drugs from the 1980s and 1990s were coming off patent, opening the door to generic competition. This so-called “patent cliff” drastically reduced revenues, forcing companies to replace lost income with new, often riskier products that were more expensive to develop.
- Complicated clinical trial designs: Clinical trials grew longer, larger, and more complex as regulators demanded broader data across more diverse patient populations. Adaptive trial designs, increased safety monitoring, and global site management created operational challenges, escalating both time and cost.
The cost and duration of clinical development steadily increased, while profit margins dwindled. The increasing complexity of trials also added significant risk to the operational success of research, both in terms of recruiting and retaining patients, and in generating the reliable results needed to support ultimate marketing approvals.
Risk-Based Monitoring (RBM) to the Rescue
By the early 2000s, it was clear that the traditional way of running clinical trials was no longer sustainable. Conventional approaches were slow, expensive, and ill-equipped to handle the increasing complexity of modern research. A new, more streamlined process was urgently needed—one that could reduce operational risk, deliver meaningful cost and timeline savings, and above all, improve data quality and protect patient safety.
Enter Risk-Based Monitoring (RBM). Once introduced, RBM quickly gained traction for its ability to prioritize oversight where it mattered most, focusing resources on critical data and processes rather than spreading efforts thin across every activity.
What Is Risk-Based Monitoring (RBM)?
RBM strategies use software, data, and analytics to monitor risk and support critical thinking and decision-making. It can improve data quality and patient safety and reduce costs by allowing Sponsors to identify and correct issues as and when they arise. Linked to Quality by Design (QbD), the model offers:
- Improved operational success rate of clinical research: By focusing resources on the most critical data and processes, RBM helps ensure that trials run more smoothly and are less likely to experience costly delays, protocol deviations, or site compliance failures.
- Higher quality: Rather than relying on retrospective checks, RBM emphasizes proactive risk identification and mitigation. This results in cleaner datasets, more reliable evidence, and higher confidence in regulatory submissions.
- Shorter timelines: Continuous monitoring and early detection of problems prevent small issues from escalating into major bottlenecks. This accelerates decision-making, reduces the need for repeat work, and ultimately shortens the path from study initiation to completion.
- Greater operational efficiency: By moving away from 100% Source Data Verification (SDV) and shifting to risk-focused oversight, RBM enables smarter allocation of resources. Monitoring teams can concentrate their time and budget on high-risk areas, cutting unnecessary site visits and administrative burden.
- Ongoing assessment: RBM is not a one-time exercise but a continuous process that adapts as new data becomes available. This allows sponsors to adjust trial execution dynamically, ensuring that monitoring strategies remain relevant throughout the study lifecycle.
- Mitigation of operational risk: With better foresight into where issues might arise—such as patient enrollment challenges, data integrity concerns, or site underperformance—RBM helps reduce both scientific and financial risks tied to trial execution.
In 2013, RBM was written into US and EU regulations, requiring Sponsors to use technology and real-time information to monitor risk proactively.
What Is Risk-Based Quality Management (RBQM)?
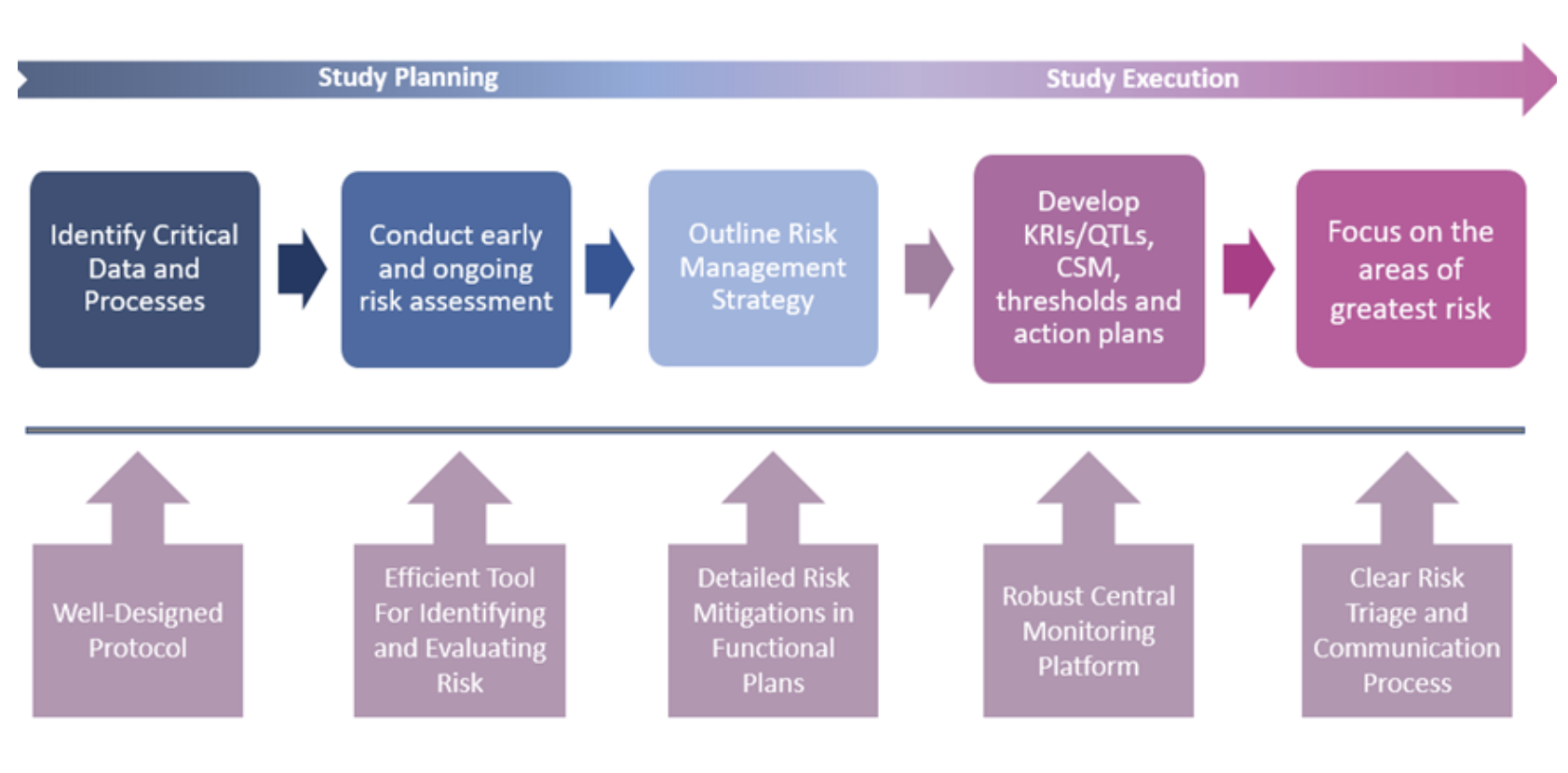
The success of the RBM model, coupled with rapid advances in clinical trial technology, paved the way for an even broader framework: Risk-Based Quality Management (RBQM). Unlike RBM, which concentrates primarily on monitoring activities, RBQM extends risk-based thinking across the entire trial lifecycle, from planning and design through execution and close-out. It shifts quality management from being reactive to proactive, embedding risk assessment and mitigation at every stage.
Since the ICH E6 (R2) guidelines of 2016 highlighted the potential of RBQM, the importance of risk-based approaches has only grown. The latest revision of the ICH E6 Good Clinical Practice guideline (R3) explicitly reinforces RBQM as the foundation for trial oversight. (R3) shifts the emphasis from retrospective quality control to proactive quality management, requiring Sponsors and CROs to integrate risk identification, evaluation, control, and ongoing review into every stage of the trial lifecycle. In doing so, ICH E6 (R3) cements RBQM as a regulatory expectation for modern clinical development.
What Is Central Statistical Monitoring?
RBQM encompasses all elements of the study, from planning right through to execution. Centralized statistical monitoring (CSM) is a critical component of the operational success of RBQM and is crucial for quality oversight. CSM interrogates all clinical and key operational data to find anomalies and discrepancies that would remain undetected by traditional techniques. It is more than just computing statistics on a subset of key variables. It is about processing all data and guiding users to where the potential issues might lie, a ‘boil the ocean’ approach to risk identification and mitigation.
Risk management underpins the overall quality of the trial by identifying, controlling, and communicating. An effective centralized monitoring approach includes Data Surveillance, Key Risk Indicators (KRIs), and Quality Tolerance Limits (QTLs).
Data Surveillance, sometimes called CSM, has been under-appreciated and under-utilized by many organizations, but it provides an effective, independent, and objective quality oversight process. While KRIs and QTLs are designed to monitor for pre-identified areas of risk, Data Surveillance or CSM can expose forms of study misconduct that may be difficult to identify or characterize during pre-study risk planning. The method can spot atypical patterns that represent potential intentional or non-intentional misconduct by running a comprehensive set of well-designed statistical tests across a broad swath of study data. It can flag issues such as fraud, sloppiness, training needs, and malfunctioning or mis-calibrated study equipment.
Key Building Blocks for Successful Risk-Based Trial Management
Starting simple creates opportunities for early wins while laying the foundation for long-term success. Key elements include:
1. Proactive Data Monitoring:
- Define which risks can be mitigated, eliminated, or accepted, ensuring clarity around decision thresholds.
- Leverage KRIs, QTLs, and CSM to detect risk signals early and remain compliant with regulatory obligations.
- Treat monitoring as an ongoing, adaptive process rather than a one-time activity.
2. Communication:
· Ensure the entire study team understands identified risks and how they are managed.
- Establish a clear triage process for escalating risks, conducting follow-up investigations, and initiating corrective actions.
- Follow the principle of delivering the right information, to the right person, at the right time—a critical driver of timely and effective decision-making.
3. Documentation:
- Maintain accurate records of all follow-up activities, corrective actions, and outcomes (e.g., via a signal and action tracker) to ensure transparency and readiness for regulatory inspection.
- Embed risk review and continuous improvement into trial operations, updating strategies as new information emerges.
It’s important to recognize that not every risk can be foreseen at the outset of a trial. This underscores the value of unsupervised data review, which helps uncover previously unidentified risks and strengthens overall trial quality.
RBQM is the Future
Risk assessment and risk management should be embedded into every trial, yet implementing RBQM can feel daunting given the volume of data and information available. The key is approaching adoption with practical, well-structured processes, the right technology, and a commitment to gradual change management.
Importantly, RBQM doesn’t need to be complicated to be effective. Its components can be introduced step by step—individually or in combination—delivering measurable impact along the way. This incremental approach allows sponsors and CROs to run better, faster, and more cost-efficient trials, while improving patient safety and data integrity.
Looking ahead, RBQM will only become more powerful. As Machine Learning (ML) and Artificial Intelligence (AI) become more integrated into clinical development, organizations can apply RBQM not just at the study level, but across entire programs and portfolios. Leveraging historical data and predictive analytics will enable smarter trial design, more accurate risk assessment, and more effective signal detection and management.
Data is knowledge, and RBQM turns that knowledge into action. Contact CluePoints to discuss.