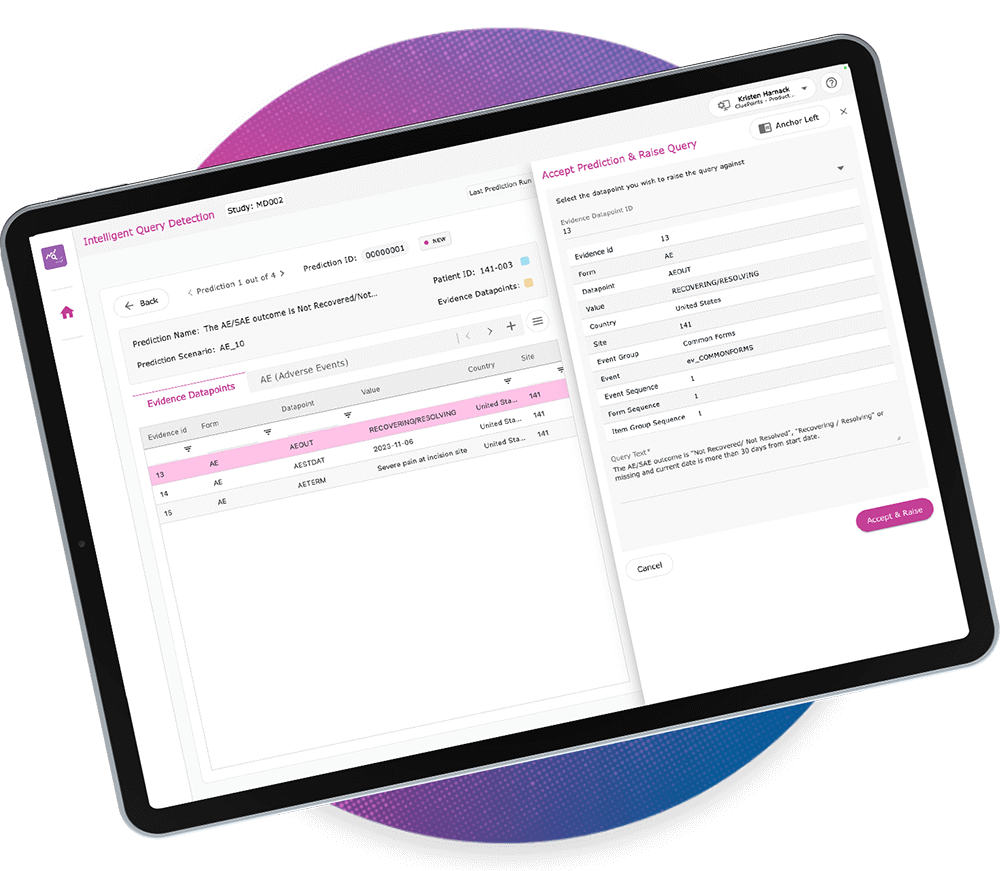
Intelligent Query Detection (IQD)
CluePoints’ IQD tool automates data discrepancy detection using high-precision, specialized large language models (LLMs). By streamlining manual query detection throughout a clinical trial, IQD enhances efficiency, accuracy, and consistency in query identification and resolution. Data Managers, who face the challenge of processing vast amounts of clinical data while ensuring query accuracy, benefit from IQD’s ability to reduce manual workload and improve oversight. With IQD, Data Managers gain a more powerful voice in driving data quality and trial success.
USERS
DE-RISKED
ISSUES DETECTED
Key Features Of The IQD Tool
Mitigate manual review listings, streamline future data cleaning, and ensure continuous data readiness with AI-powered solutions.

Adaptive Learning Model
Ensure ongoing accuracy in identifying and resolving data issues by continuously refining the LLM through user interaction.

Expandable Across Trials
Identify data anomalies across trials, seamlessly handling diverse naming conventions and protocols—no custom programming required.

High-Precision Accuracy
Minimize false positives in manual query detection, using advanced LLMs to achieve superior precision in identifying data anomalies.

Automated Query Suggestions
Raise queries faster with suggested text for consistent communication, reducing duplicate queries and speeding up resolution.

EDC System Integration
Ensure data discrepancies reach the right person promptly. IQD seamlessly integrates with EDC systems to raise and manage queries in one platform.

Intuitive User Interface
View and share suggested queries with supporting evidence, streamlining communication and resolution.
Streamlined Query Processes
IQD automates data discrepancy detection, ensuring efficient query communication and resolution. By reducing time spent manually reviewing data, Data Managers can focus on higher-priority tasks and accelerate issue resolution across sites. This leads to cost savings, faster trials, and stronger regulatory compliance.
Higher Precision, Fewer False Positives
IQD’s advanced LLMs accurately detect data discrepancies, significantly minimizing false positives and reducing the burden of unnecessary queries. With cleaner data across sites and less manual effort, teams can maintain trial integrity and ensure higher-quality regulatory submissions.
Lower Query Costs
Automated query detection enables users to raise queries with just a few clicks, reducing resource strain and improving efficiency. By streamlining query generation and anomaly detection, IQD frees up resources for more strategic, high-value tasks.
Consistent & Clear Query Descriptions
IQD suggests standardized query text, reducing redundancy and ensuring clear, consistent communication with sites. This minimizes miscommunication and back-and-forth exchanges, enabling faster query resolution and strengthening site relationships.
Smarter, Automated Query Listings
IQD eliminates manual programming by leveraging AI-driven models that continuously improve at detecting and highlighting data discrepancies. Automating query listings frees Data Managers to focus on data quality oversight instead of manual updates.

Explore Our Knowledge Center
IQD for Every Clinical Trial
CluePoints provides Sponsors and CROs with a smarter way to automate data discrepancy detection and streamline future data cleaning. By leveraging the potential of AI using advanced statistics and ML, CluePoints is transforming a powerful, best-in-class platform of solutions into positive clinical development outcomes as our partners work to develop innovative therapies to improve the lives of people worldwide.
Contract Research Organizations (CROs)
Pharmaceuticals
Medical Devices
Academic Community
Gain an Edge.
Get Involved.
More to Come.
Clinical data is growing at an unprecedented scale with more Volume, Variety, Velocity, Veracity, and Value than ever. Yet, traditional query detection lags behind, leaving Data Managers buried in manual work. IQD changes that. Be among the primary adopters shaping the future of clinical data oversight. Join us to gain early access, provide feedback, and explore what’s coming next.