Many clinical trial Sponsors and Contract Research Organizations (CROs) realize the great benefits of Risk-Based Quality Management (RBQM), and leading regulatory bodies such as the FDA, EMA, and ICH are championing its progressive strategy, including Risk-Based Monitoring (RBM) in clinical trials. RBQM advocates for a shift toward centralized and risk-focused monitoring methods, which aim to optimize resource allocation, enhance data quality, and improve trial efficiency by concentrating monitoring efforts on areas with the highest potential impact on patient safety and the reliability of trial results.
A Tufts Center for the Study of Drug Development (CSDD) survey found that RBQM elements were deployed in 55 percent of clinical trials.1 However, some organizations still face challenges in identifying an approach to best implement the transformative approach in their overall and ongoing clinical research strategy. As the leading authority in RBQM, CluePoints is well-equipped to give you the rundown—whether you’re shaping an RBQM strategy, aligning with the latest industry guidelines, or refining tactical monitoring approaches.
RBQM Components & Considerations
There’s a lot to think about, from setting the right objectives to ensuring you have the right people, processes, and technology in place. Here are some essential considerations for successfully implementing RBQM according to your business needs.
RBQM focuses on managing quality in clinical research by avoiding “errors that matter” rather than striving for zero errors. While perfection is unattainable, the priority is to resolve errors that could impact patient safety or data reliability. The core of this approach is “risk-based,” which doesn’t imply accepting increased risk in research. Instead, it involves actively assessing and mitigating risk by understanding what could go wrong in a study and taking steps to reduce, eliminate, or control these risks.
Quality by Design (QbD) is an essential component of RBQM. QbD involves proactive identification and assessment of critical-to-quality (CtQ) factors and associated risks during the design of study protocols to optimize clinical trial quality and operational success. QbD and RBM are both phases of RBQM, focused on improving operational success through iterative risk assessment and mitigation.
To succeed with a risk-based model, we need flexible, connected technology. Current systems fail to enable collaboration, standardization, and meaningful data insights. Interoperable platforms can bridge this gap, improving collaboration among suppliers, partners, and Sponsors while simplifying data sharing and unification. Open, adaptable systems provide the flexibility to customize technologies for each study, making them essential for hybrid trials where patient and operational needs constantly change.
Where You Are in Your RBQM Journey Matters
The industry’s adoption of RBQM presents a varied picture, with Sponsors and CROs of different sizes and specialties exploring diverse approaches. Larger companies face complexities in making RBQM a standard practice across all studies, while smaller organizations often implement RBQM on a program-by-program basis. Wherever you are across the clinical trial lifecycle—whether in trial design, execution, or inspection readiness—you can start implementing components of RBQM effectively.
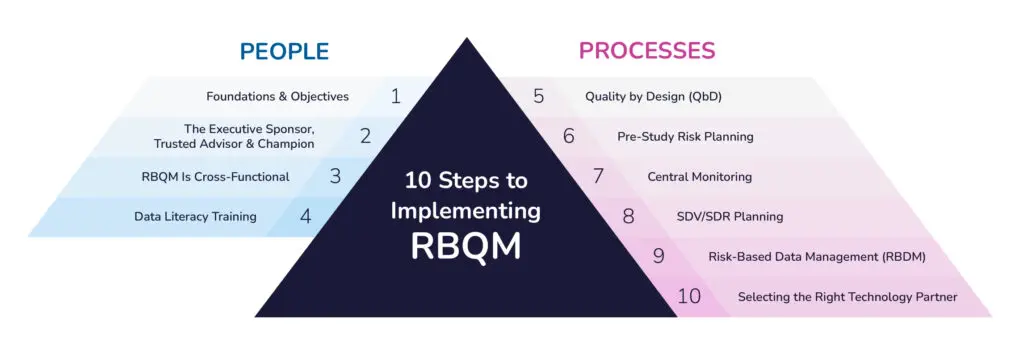
10-Step Process for Implementing RBQM in Clinical Trials
Clinical trial organizations are acknowledging the advantages of RBQM and making moves for more widespread implementation—let’s start with getting your people in place and on board.
-
Foundations & Objectives
Defining and quantifying objectives is fundamental. Vague aims like “improving data quality” will limit the ability to measure progress and take course-correcting action proactively. Further interrogation will uncover more concrete goals. Definitive, quantifiable objectives, backed by all stakeholders, are the secret to alignment and success with your RBQM-related goals. These may include enhanced quality, increased efficiency, or shorter cycle times.
-
The Executive Sponsor, Trusted Advisor & Champion
Securing the right people is crucial, with three roles as the foundation. First, a senior executive sponsor who strongly supports RBQM will clearly set the tone for the entire organization that RBQM is the path forward. Second, trusted advisors will be important to help shape processes and mentor team members. Finally, RBQM champions within the organization will drive change through effective communication and motivation. Depending on the size and setup of your organization, it may be possible that one capable individual can take on more than one of these three roles.
-
RBQM Is Cross-Functional
Forming a diverse cross-functional team and ensuring cross-functional ownership of RBQM is essential for its ultimate success. This team should typically include stakeholders from safety, site management, data management, biostatistics, and medical/clinical, among other departments. Sponsors, CROs, and RBQM technology providers are also often involved. This ensures a complete perspective in risk management.
-
Data Stewardship & Literacy Training
Data stewardship is key to managing knowledge effectively. To do this right, we must understand how data is gathered, organized, analyzed, and used. Ensuring that the RBQM team is well-versed in data literacy is vital for effective decision-making. This consideration applies to both Sponsors and CROs, emphasizing the importance of understanding data origins and sources and mastering multiple data sets for informed decision-making. By combining stewardship with knowledge management, we can record the risks we find and use them to improve future study designs.
You have your team. Now, it’s time to turn to the processes.
-
Quality by Design (QbD)
RBQM starts by optimizing study quality directly in the design of your trial by applying several key QbD principles. First, proactively solicit input and feedback from both the patient community and relevant medical and investigative site communities. Second, iterative risk assessments should be conducted during protocol design. This should look largely similar to your pre-study risk planning, in which a cross-functional team identifies and addresses key risks associated with CtQ factors. Third, the focus should be on reducing complexity wherever possible in trial design by critically assessing the need for proposed trial assessments and procedures.
-
Pre-Study Risk Planning
Once the trial protocol is finalized, your study team should conduct a fresh risk assessment to confirm and address the key remaining risks. The objective here is less about optimizing trial design and more about optimizing operational processes, including quality oversight and monitoring. Simplify oversight by consolidating all risks into a cohesive plan to align operational and monitoring teams. Address site risks (e.g., aging queries, adverse events), subject-level risks (e.g., protocol deviations), and trial-wide risks (e.g., QTLs). Guide the study team with a critical question: “What can go wrong?” Focus on CtQ factors to frame discussions and ensure decisions are practical and data-driven. Data shows that 80% of trials implemented at least the initial risk assessment, and 78% implemented the ongoing risk assessment in 2021, while the implementation of the other six RBQM components ranged from 22–43%.2
-
Central Monitoring
During study execution, effective centralized monitoring should be comprised primarily of statistical data monitoring (SDM), Key Risk Indicators (KRIs), and Quality Tolerance Limits (QTLs). Focus on a core set of KRIs to detect early risks and minimize false alerts while prioritizing only the essential study-level risks for QTLs. Implement SDM for comprehensive quality oversight, detecting study misconduct that KRIs and QTLs miss.
-
SDV/SDR Planning
Once you confirm site compliance through source review of the first patients per site, you can significantly reduce reliance on source data verification and review (SDV/SDR). Periodic sampling will be sufficient, prioritizing critical patient data. Apply a consistent SDV/SDR plan per site initially, expanding only as needed to investigate specific risks or issues.
-
Risk-Based Data Management (RBDM)
Aligned with RBQM principles and similar to a more targeted approach to on-site monitoring and SDV/SDR, your data management team should embrace a more intelligent, targeted approach to data review and data cleaning. Focus reviews and data checks on critical data and leverage the increasing power of AI-driven solutions to detect data discrepancies efficiently and automatically code adverse events and concomitant medications.
-
Selecting the Right Technology Partner
The right technology partner is pivotal to successful RBQM. We advise identifying a comprehensive, configurable solution with the necessary functionality to support your defined outcomes. Moreover, try to avoid technologies that require extensive build and management.
Grab our tip sheet to learn, share, and implement RBQM strategies with confidence—download now and take the first step toward transforming your organization.
RBQM Helps Transform Clinical Trials
CluePoints provides Sponsors and CROs with a smarter way to detect and manage risks that could impact clinical trial outcomes. By leveraging the potential of AI using advanced statistics and machine learning, CluePoints is transforming a powerful, best-in-class platform of solutions into positive clinical development outcomes as our partners work to develop innovative therapies to improve the lives of people worldwide.
The transformative potential of Risk-Based Quality Management (RBQM) in clinical trials is clear. While the industry acknowledges the importance of mitigating risks and improving data quality through RBQM, there’s room for increased adoption. As the ICH E6 (R3) guidance approaches, organizations are gearing up for comprehensive risk management plans, anticipating a pivotal moment in RBQM adoption. The discussion remains open for professionals to share experiences and thoughts on current best practices, enriching the ongoing discourse in the pursuit of successful RBQM implementation. And CluePoints remains available to help you answer the question of “how” rather than “if.”
Want to learn more? Download “The Ultimate Guide to RBQM,” and contact us to see how we can help your business implement RBQM for success.
References
- Miessler, James. “RBQM Adoption Still Limited in Clinical Trials, New Data Show.” Center Watch, 2023. https://www.centerwatch.com/articles/26645-rbqm-adoption-still-limited-in-clinical-trials-new-data-show
- Adams, A., A. Adelfio, B. Barnes, R. Berlien, D. Branco, A. Coogan, L. Garson, N. Ramirez, N. Stansbury, J. Stewart, G. Worman, P. J. Butler, and D. Brown. 2023. “Risk-Based Monitoring in Clinical Trials: 2021 Update.” Therapeutic Innovation & Regulatory Science 57, no. 3 (May): 529–37. https://doi.org/10.1007/s43441-022-00496-9. Published online January 9, 2023. PMID: 36622566; PMCID: PMC9829217.