AI Solutions Supported by Clinical Trial Expertise & Client-Focused Support Services
CluePoints offers comprehensive client-focused support throughout the clinical trial lifecycle through seasoned industry data analysts and RBQM specialists who can help pinpoint where to concentrate active efforts while handling RBQM implementation and configuration duties. With a focus on meticulous data exploration, our team stands ready to streamline your clinical trial workflow and provide a solid foundation for regulatory endeavors.
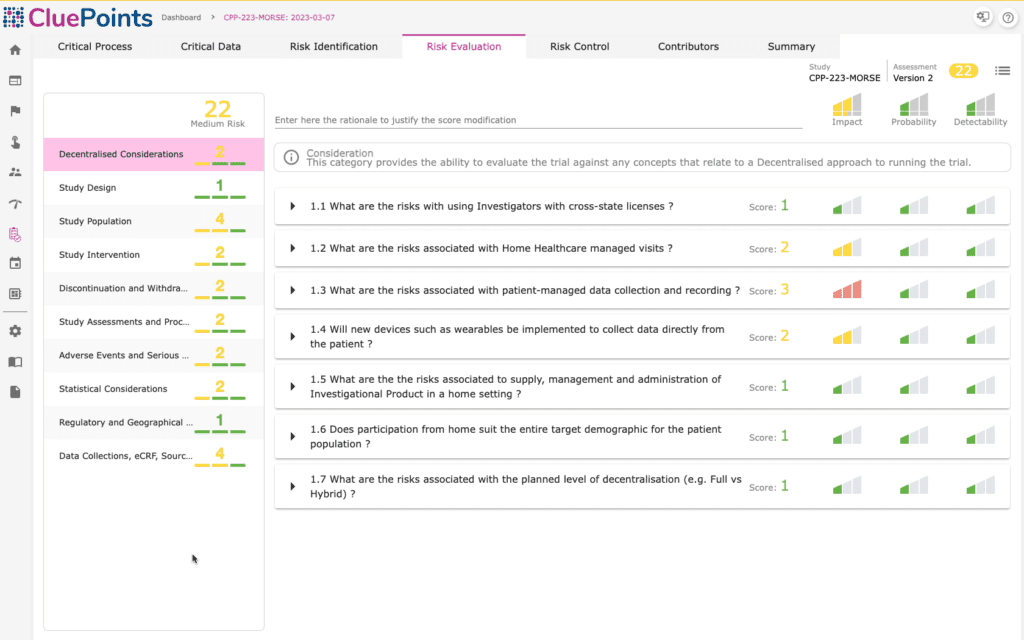
Central Monitoring
CluePoints bolsters proposed and ongoing clinical trials with comprehensive RBQM support. Our team of seasoned industry data analysts will expertly configure the system on your behalf, meticulously processing your clinical trial and operational data to deliver insightful findings directly to you. We help pinpoint precisely where to concentrate active efforts, optimize clinical data quality, and secure the success of your trial.
RBM/RBx Implementation
As the leading authority in risk-based execution (RBx), CluePoints can guide your journey to RBQM technology implementation success. Our dedicated professional services team stands ready to assist you every step of the way. Whether it’s shaping your RBx strategy, aligning with the latest FDA and ICH guidelines, or refining tactical monitoring approaches and key risk indicators (KRIs), rely on the wealth of historical experience we bring to the table.
Site Inspection Readiness
Is your clinical trial truly complete if you lack comprehensive visibility into site performance and data quality? Are you confident that your clinical trial data will withstand regulatory scrutiny without any surprises? Our RBQM experts address these concerns head-on. By meticulously analyzing all your clinical and operational data, we identify sites at risk of inspection, empowering you to prepare and mitigate potential issues before they arise.
Mergers & Acquisitions
Are there hidden pitfalls lurking within the clinical data backing your proposed acquisition, jeopardizing its viability for a successful regulatory submission down the line? CluePoints offers the expertise to thoroughly scrutinize your clinical trial data for any irregular patterns or notable absences that could signal underlying issues. Our rigorous testing helps validate data integrity and associated process efficacy, ensuring a solid foundation for regulatory endeavors.


