Our RBQM innovators leverage cutting-edge advancements in statistics and machine learning to develop AI-powered solutions that transcend traditional data analysis. By adhering to FDA and ICH guidelines, we accelerate result delivery to study teams, facilitating prompt issue resolution. Our RBQM technology illuminates outliers and anomalies in real time, offering users a better way to identify, visualize, manage, and document trial risks.
Empower proactive risk management by pinpointing vulnerable areas, facilitating informed decision-making, and ensuring compliance throughout clinical trials.
Rank sites based on performance and tolerance metrics, uncovering ‘at-risk’ locations in need of proactive corrective action.
Identify systematic issues, monitor key metrics, and document deviations from pre-defined QTLs to ensure accountability and compliance throughout trials.
Test all collected data, including both clinical and operational, exposing atypical data patterns that indicate operational challenges.
Generate a priority listing of patient pairs by specifying configurable variables likely to indicate duplicates and review based on side-by-side scoring and evaluations.
Flag atypical patient patterns, prioritize investigations into anomalies within data sets, and target those of utmost importance.
Explore clinical and operational data from various perspectives and domains in customizable formats with multiple data widgets.
Determine vulnerable trial areas, define risk controls, and monitor capabilities to detect evolving threat levels during study execution.
Assess identified issues, annotate commentary throughout signal processing, deploy automatic auditing, and leverage extensive exporting capabilities.
Analytics underpins RBQM by providing the means to analyze data, detect patterns, and derive insights that drive proactivity and compliance. Our RBQM solutions ensure effective risk and data management, as well as the optimization of clinical trial processes, ultimately leading to more reliable results and improved patient outcomes.
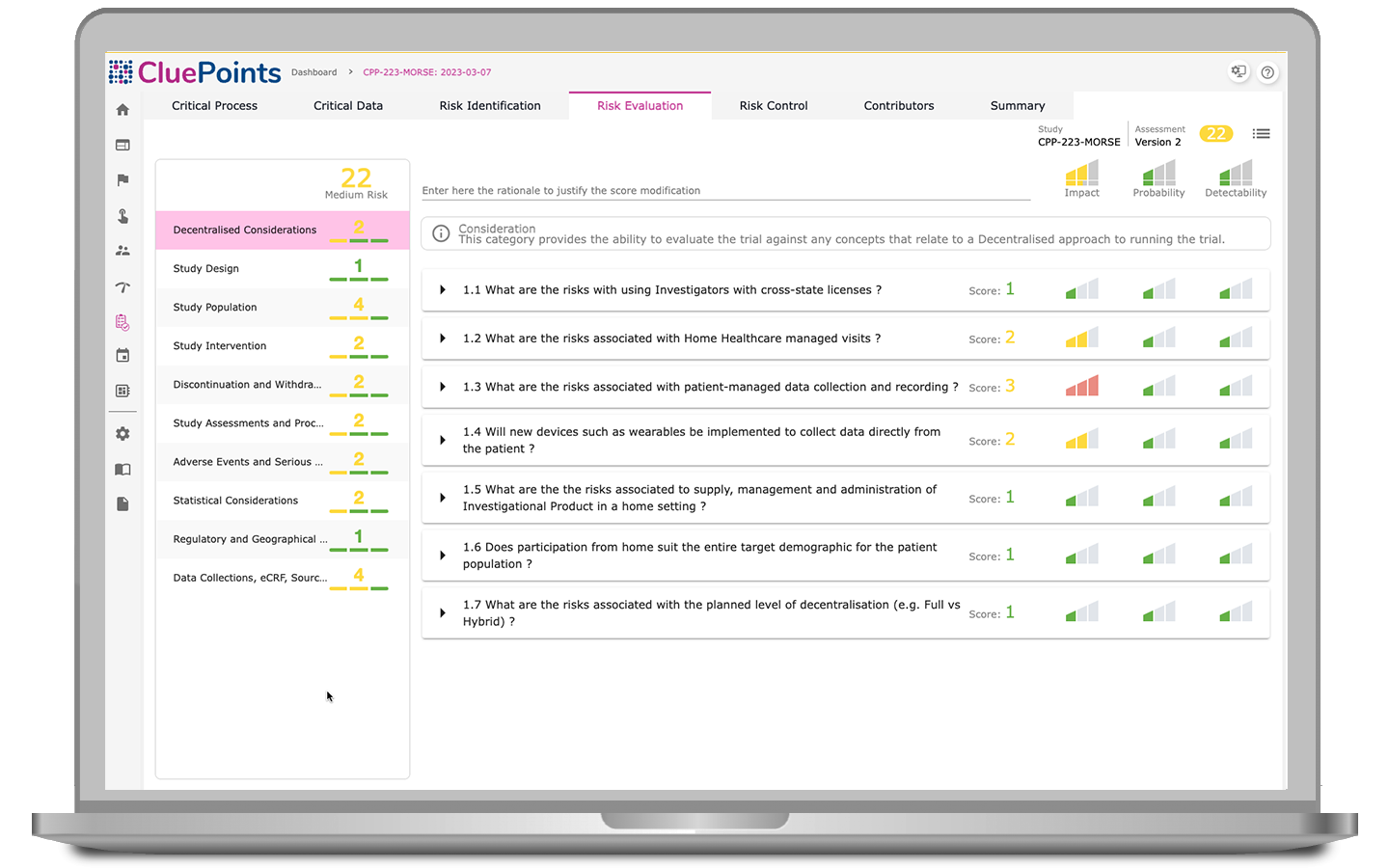
The documentation of issues and actions taken in clinical trials is essential for managing risks effectively and ensuring regulatory compliance. Our RBQM solutions foster organization-wide transparency and facilitate the knowledge transfer of all crucial analytics and risk management processes.
CluePoints provides extensive assistance throughout the clinical trial process by deploying experienced industry data analysts and RBQM specialists. They pinpoint areas requiring active attention and manage implementation, streamlining workflows and establishing a robust foundation for regulatory compliance efforts.

CluePoints provides our community with smarter ways to run clinical trials. By leveraging the power of AI using advanced statistics and machine learning, CluePoints’ customers and partners can harness its proven, best-in-class platform of solutions to develop new therapies to improve the lives of people worldwide.
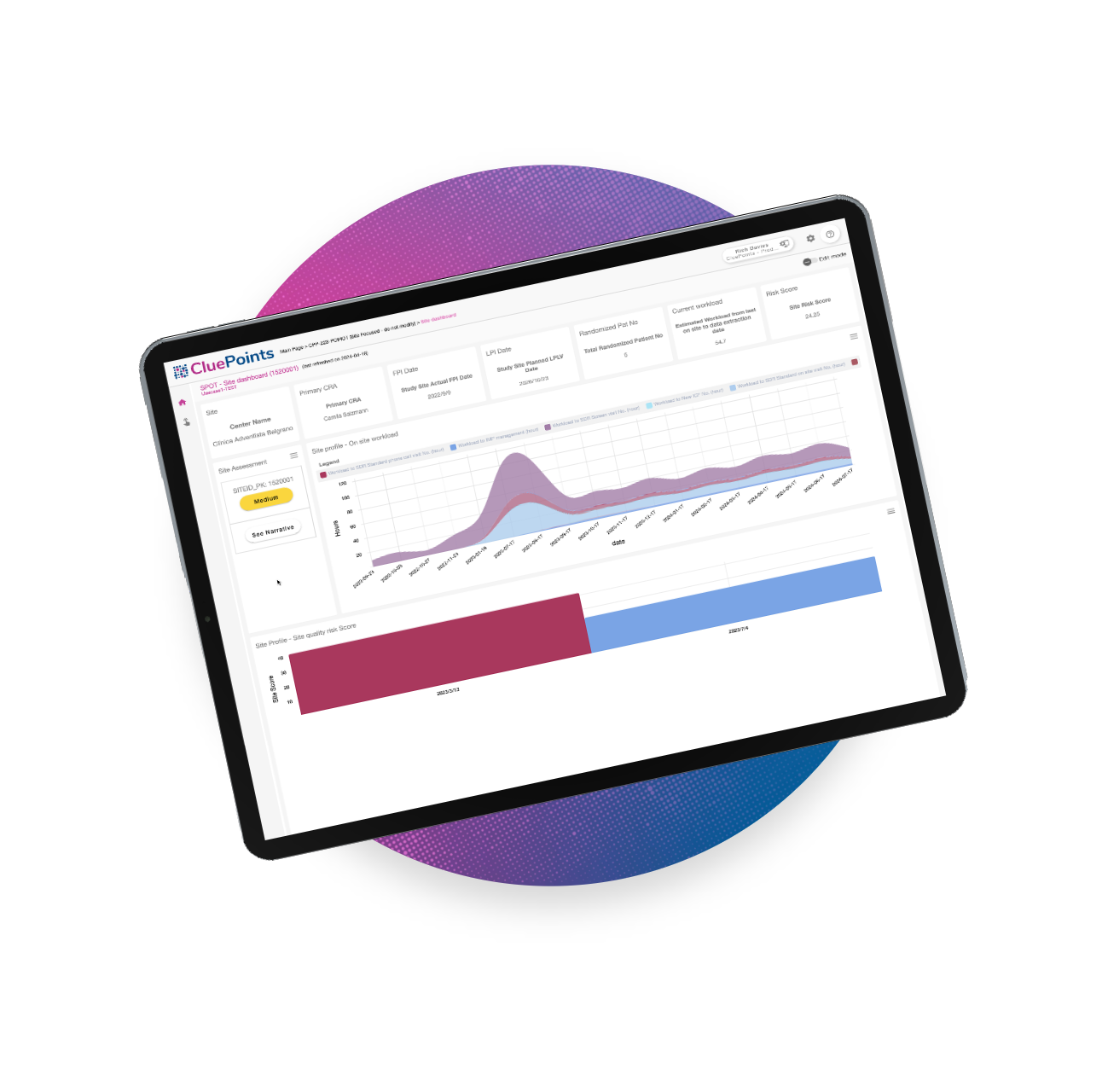
In addition to RBQM, CluePoints provides SPOT, which enables adaptive site monitoring for swift anomaly identification and actionable insights, improving study performance evaluation, site visit planning, and risk assessment. Pair with RBQM or leverage it as a solo tool—it’s your choice.
CluePoints is dedicated to revolutionizing clinical trials through AI, advanced statistics, and machine learning. Aligned with global regulatory guidance, our holistic risk-based approach is designed to drive positive outcomes. Ready to uncover the clues and pinpoint success in your next trial? Contact our expert team today.
"*" indicates required fields
Site Profile & Oversight Tool (SPOT) and Intelligent Medical Coding (IMC) unveiled by RBQM innovator at DIA Global Annual Meeting 2024 King of Prussia, PA –