A review of FDA marketing submissions between 2000 and 2012 revealed roughly one- third (32%) of all first-cycle review failures (16% of submissions overall) were driven by quality issues. Why was this? While the pharmaceutical industry had enjoyed economic growth in the 1990’s, drug makers started to face growing pressure at the turn of the century from multiple directions:
- Highly publicized safety issues with marketed drugs
- A slowing of innovation
- Patent expirations
- Complicated clinical trial designs
The cost and duration of clinical development steadily increased, while profit margins dwindled. The increasing complexity of trials also added significant risk to the operational success of research, both in terms of recruiting and retaining patients, and in generating the reliable results needed to support ultimate marketing approvals.
Risk-Based Monitoring to the Rescue
The traditional way of conducting trials was not fit for the 21st century and a more streamlined and efficient process was needed to reduce risk while offering significant cost and timeline benefits. Improving data quality and patient safety is a key concern, in addition to controlling the spiralling costs of drug development research. Risk-Based Monitoring (RBM) was thrown into the spotlight. The model’s success, combined with advances in clinical trial technology, has seen the approach extended to cover the whole trial execution; cue ‘RBQM’ (Risk-Based Quality Management). But what is the difference between RBM and RBQM, what are the benefits, and how can sponsors and CRO’s harness the power of risk-based trial management?
What is Risk-Based Monitoring?
RBM strategies use software, data and analytics to monitor risk and support critical thinking and decision making. It can improve data quality and patient safety as well as reducing costs by giving sponsors the ability to identify and correct issues as and when they arise. Linked to Quality by Design (QbD) the model offers:
- Improved operational success rate of clinical research
- Higher quality
- Shorter timelines
- Greater operational efficiency
- Ongoing assessment
- Mitigation of operational risk
In 2013, RBM was written into US and European regulations, requiring sponsors to use technology and real-time information to proactively monitor risk.
What is Risk-Based Quality Management?
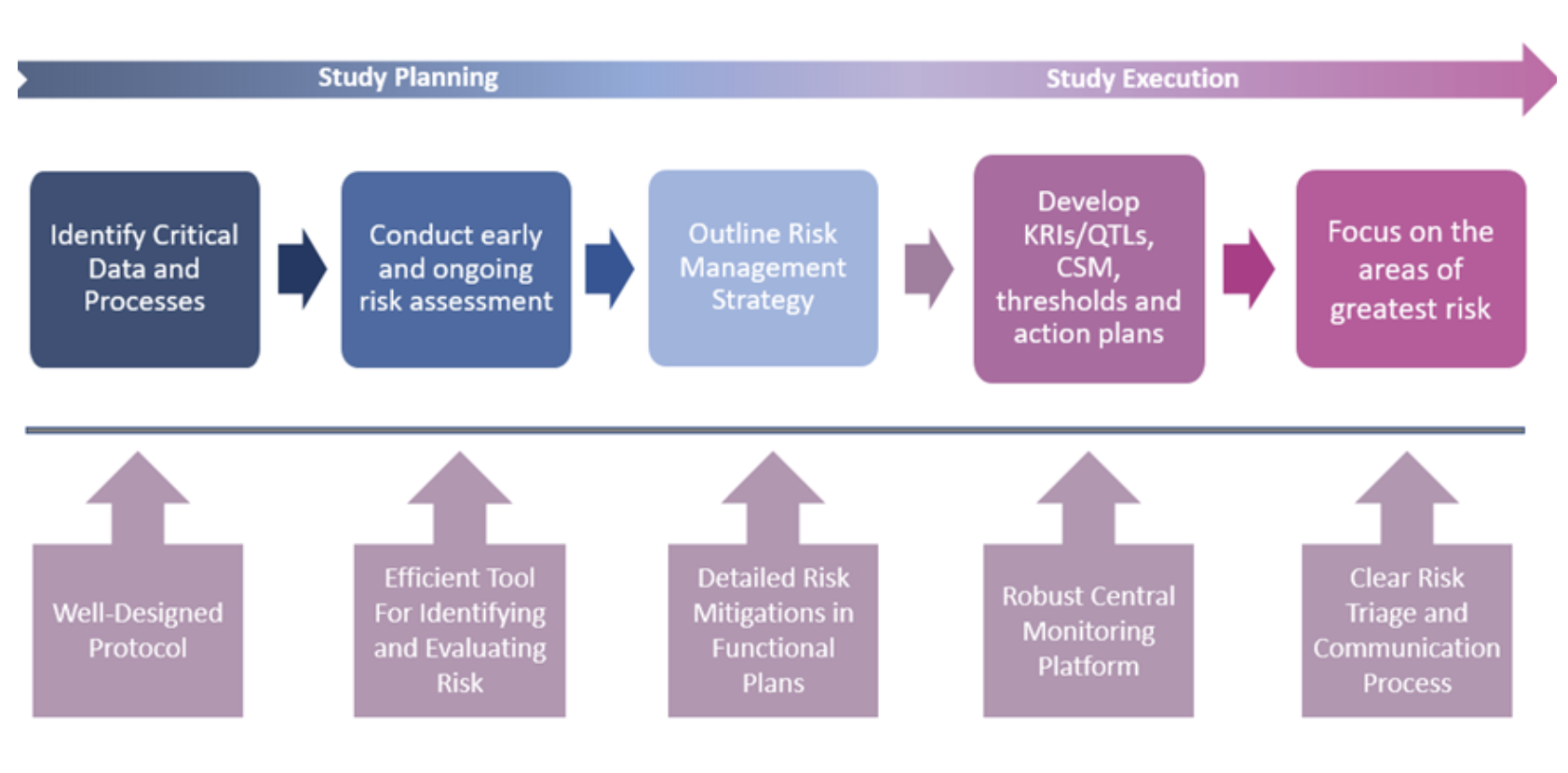
RBQM applies the principles of RBM to all areas of quality management. The latest version of the GCP quality standard extends this approach to every aspect of study execution. Indeed, the ICH E6 R2 guideline outlines key factors that have emerged over the past 15 to 20 years which have led to this shift, including the use of electronic and digital technologies (EDC, ePRO, IRT). The move away from paper has opened a tremendous opportunity to plan and manage clinical research more effectively and efficiently. Sponsors and CROs are now embracing RBQM to address the growing crisis in research complexity, duration and cost.
What is Central Statistical Monitoring?
RBQM encompasses all elements of the study, from planning right through to execution. Centralized Statistical Monitoring (CSM) is a critical component of the operational success of RBQM, crucial for quality oversight. CSM interrogates all clinical and key operational data to find anomalies and discrepancies that would remain undetected by traditional techniques. It is more than just computing statistics on a subset of key variables. It is about processing all data and guiding users to where the potential issues might lie, a ‘boil the ocean’ approach to risk identification and mitigation.
Risk management underpins the overall quality of the trial by identifying, controlling and communicating. An effective centralized monitoring approach includes Data surveillance, Key risk indicators (KRIs) and Quality tolerance limits (QTLs).
Data Surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but it provides an effective independent and objective quality oversight process. While KRIs and QTLs are designed to monitor for pre-identified areas of risk, Data Surveillance or CSM can expose forms of study misconduct that may be difficult to identify and/or characterize during pre-study risk planning. By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. It can flag issues such as fraud, sloppiness, or training needs, as well as malfunctioning or mis-calibrated study equipment.
Elements for Success
Starting simple enables quick wins and longer-term overall success. Crucial steps include:
Proactive Data Monitoring:
- Identify what is possible to mitigate, eliminate and accept.
- Key Risk Indicators (KRIs), Quality Tolerance Limits (QTLs) and Central StatisticalMonitoring (CSM) are all crucial to the process to identify risk signals and comply with the regulatory obligations.
Communication:
- The entire study team should be aware of the risks and how they are being managed
- Establish a triage process for communicating risks, follow up investigations, andcorrective actions.
- The right person, the right information, at the right time is the best approach to decide how and when to act.
Documentation:
- Accurately record follow-up, corrective actions and outcomes (signal and action tracker) for regulatory inspection purposes.
- Risk review and continuous improvement should be maintained throughout the trial.
Note that every risk may not be identified initially, highlighting the importance of “unsupervised” data review.
RBQM is the Future
Risk assessment and risk management should be part of every single trial yet RBQM implementation can be overwhelming for an organization given the wealth of information that is currently available. Successful RBQM rollout is achieved by applying thoughtful but simple processes, smart technology, and a focus on evolutionary change management.
All the key components of RBQM implementation do not need to be complex to be effective. Elements of RBQM can be implemented individually and independently to great success, making clinical trials better, faster and cheaper for sponsors and CROs – and safer for patients.
Looking to the future, RBQM can also be applied across studies, programs and organizations alongside machine learning and artificial intelligence to identify program, organization or industry trends. Learning from historical data can help make future decisions to support study setup, risk assessment, analytical tools and signals management. Data is knowledge.