COVID-19 Risk Planning
As advised in the coronavirus guidances recently issued by FDA, EMA, and MHRA, it is important to perform a fresh risk assessment for each study to identify and mitigate risks pertinent to the COVID-19 crisis. Your organization is likely already aware of key challenges that will be common to most if not all clinical studies, such as the following:
- Increase in missed patient visits
- Increase in missed assessments even for patient visits that occur
- Increase in missed study treatments, for various reasons including missed patient visits and IP supply chain disruptions.
- Increase in patient early terminations (“study drop-outs”)
- Difficulty recruiting new patients
- Slower site responsiveness, including delays in EDC data entry and query response.
- Gaps in reporting of adverse events, especially to the extent that sites will have less frequent interactions with their patients.
- Less frequent on-site monitoring visits
However, the relative scope and impact of these common risks will vary by study, as will the proposed mitigations. Additional study-specific risks may also need to be addressed. For example, the increased threat of Coronavirus infection may pose a particularly high risk for certain patient populations and/or study treatments (e.g., studies testing immunosuppressant therapies).
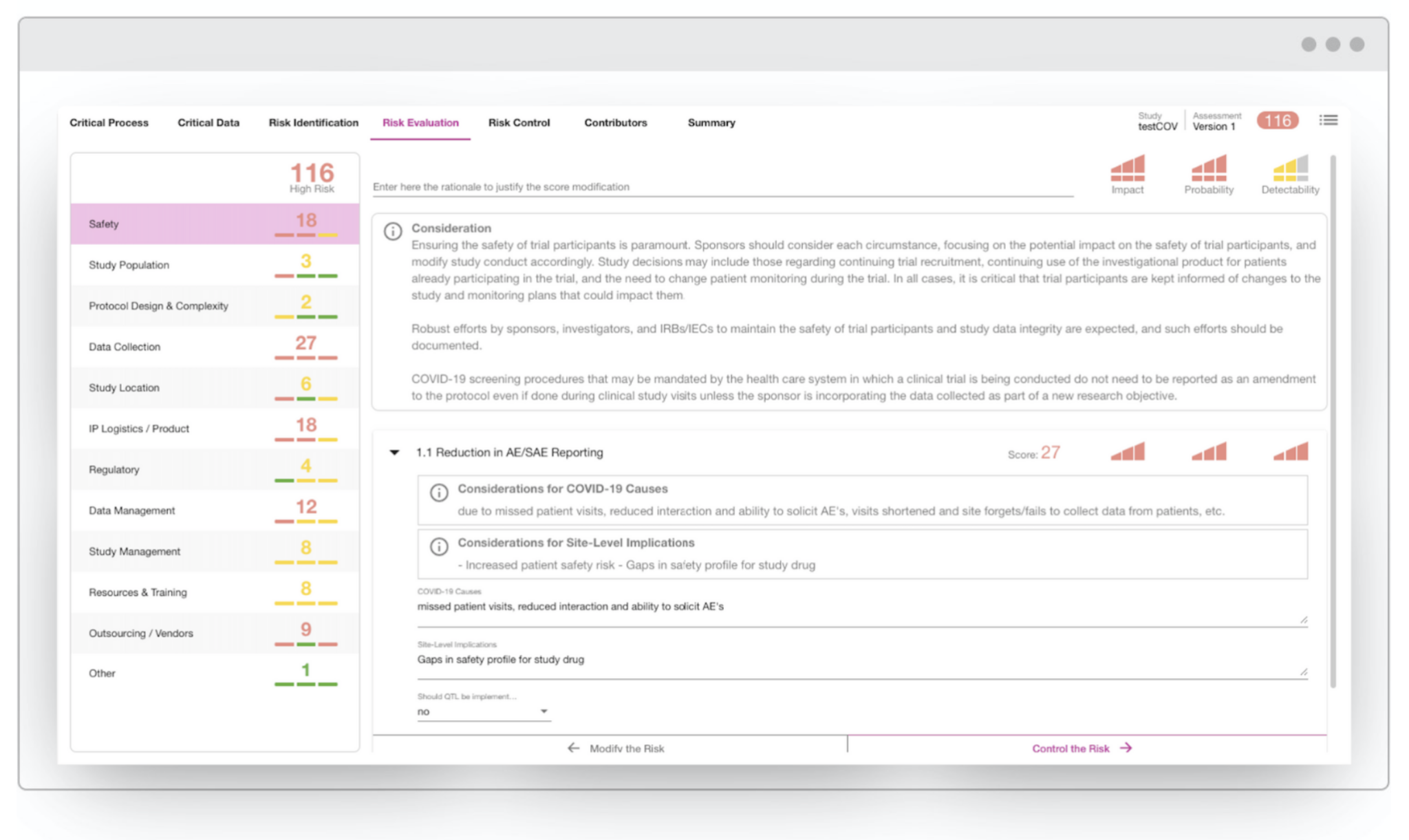
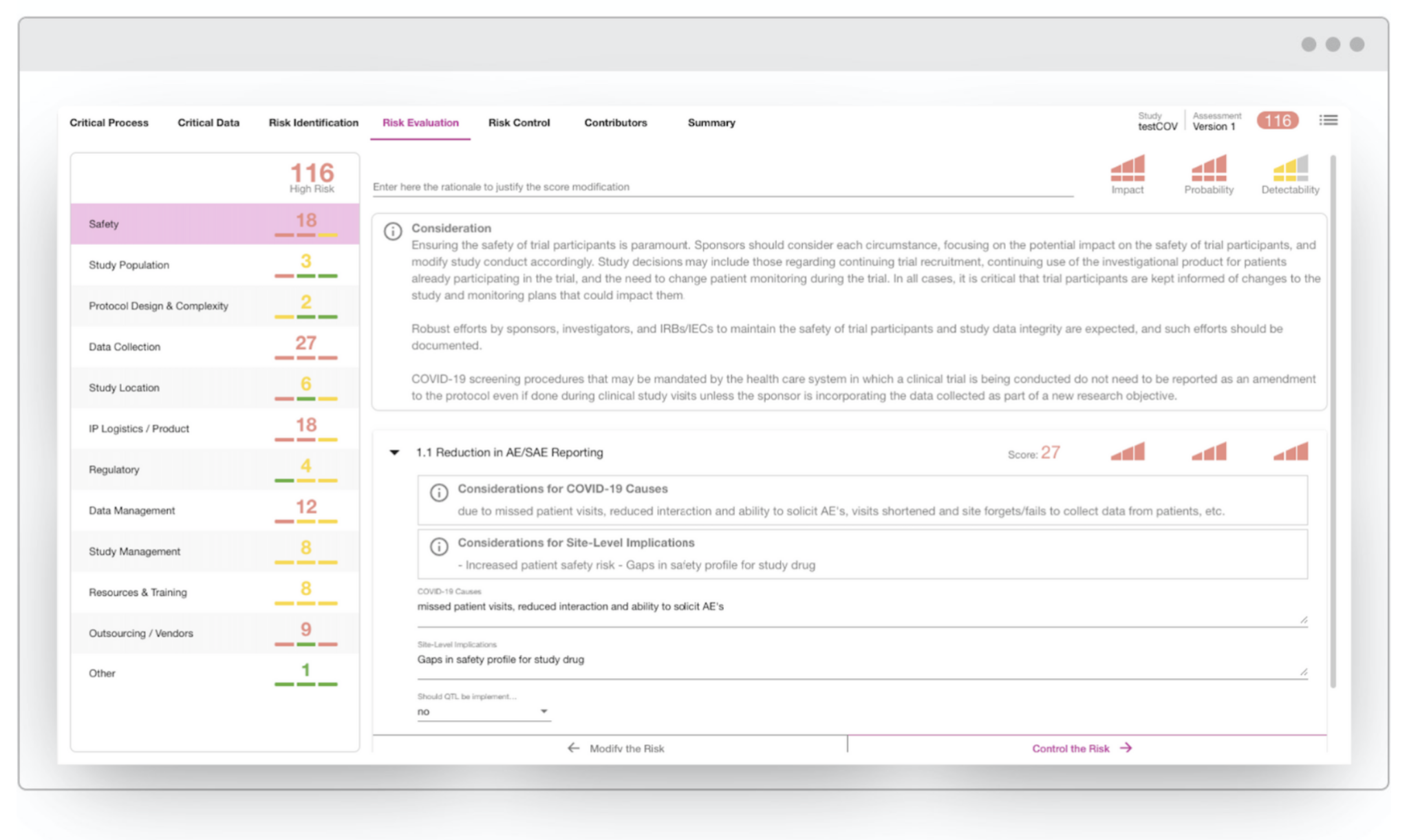
The CluePoints platform includes a mature online Risk Assessment solution that can be leveraged for performing risk assessment and mitigation planning during this crisis. Our team of experts have also developed a special coronavirus-specific Risk Assessment template that includes a set of relevant risk categories and associated considerations gleaned from the regulatory guidance documents issued by FDA, EMA and MHRA.
The image below illustrates this CluePoints COVID-19 Risk Assessment template – click the image to enlarge.
Here’s what you will Receive from CluePoints if you Request our Support
- Free access to CluePoints COVID-19 specific templates on the CluePoints Risk Assessment and Categorization Tool (RACT) for the duration of the crisis
- Access to simple online eLearning (30 mins) to allow rapid RACT set-up
- Support via CluePoints Subject Matter Experts (SMEs) to facilitate onboarding
How to get Access to the COVID-19 Risk Assessment Package
Getting started couldn’t be easier. Click the button below to send your details and one of our experts will follow up with you to get you set up with the COVID-19 Risk Assessment Package.