From RBM to Risk-Based Quality Management
Before, it was about Risk-Based Monitoring. Now, it’s about Risk-Based Quality Management (RBQM), an ICH and FDA-advocated approach to managing risk for the entire clinical trial lifecycle. CluePoints provides the enabling technology and expertise to drive this new paradigm.
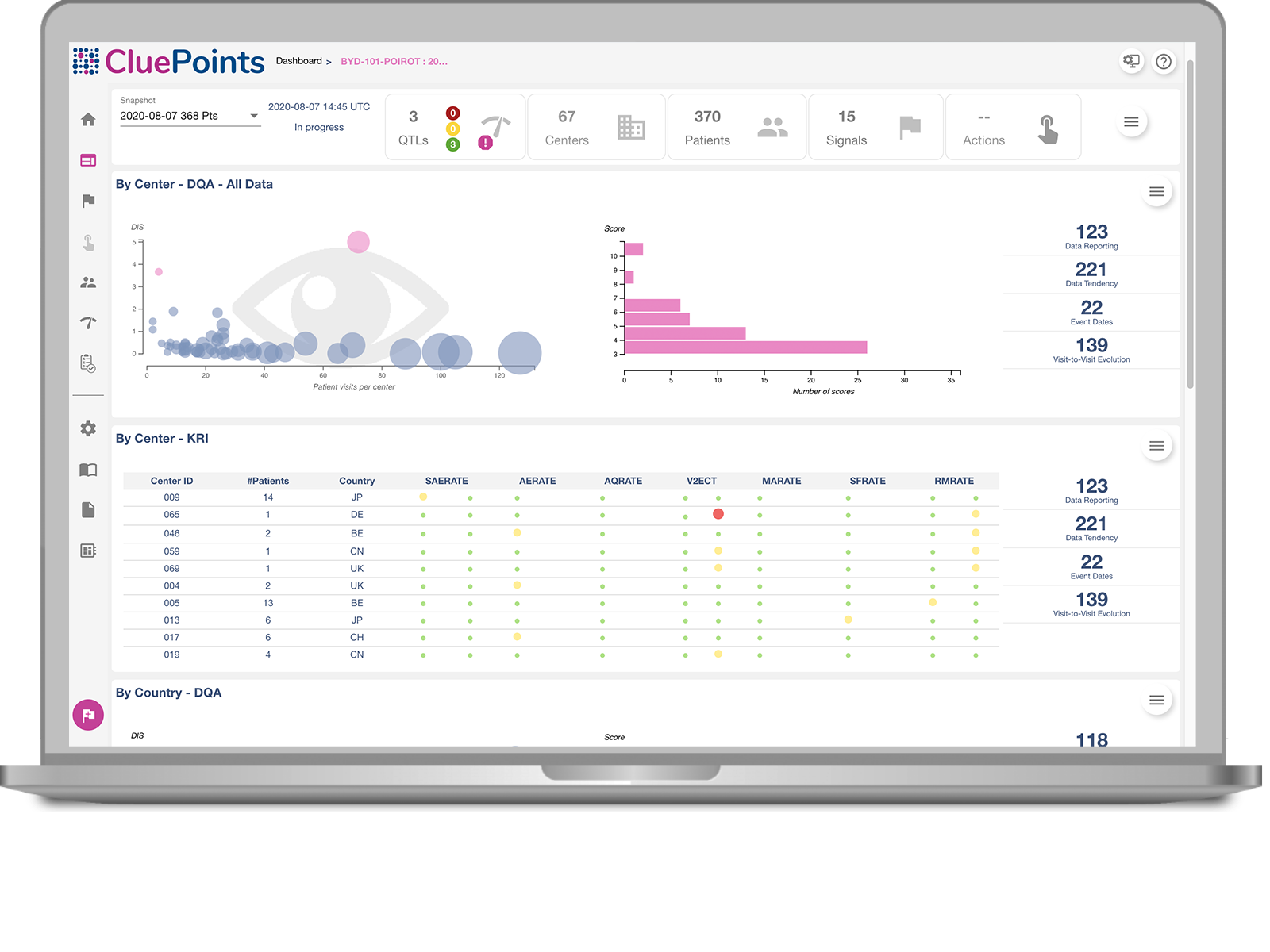
Quality Tolerance Limits
Study or Patient Safety Risk using Quality Tolerance Limits.
Key Risk Indicator Dashboard
Site Risk using study-specific Key Risk Indicators.
Data Quality Oversight
Discovery of unknown issues through statistical interrogation of all data.
Issue Tracking
Integrated Issue and Action Management to manage resolutions.
Hover over the toggles to discover the features.
Got a Question?
Whether you’re looking to get started or need support, we’re here to help!
Explore our
Risk-Based Quality
Management
Software
CluePoints offers an array of solutions designed to help Sponsors and CROs quickly and efficiently identify, manage and document risks for the entire study lifecycle and ultimately, to drive compliance with ICH E6 (R2).
Explore our
Risk-Based Quality
Management
Services
Our Subject Matter Experts boast unrivaled experience in the data quality oversight arena and can help you translate your quality oversight goals into a detailed strategy with a pragmatic roadmap for delivery.
