Risk Assessment and Mitigation
Questionnaire
Implement Risk Assessment specific to your trial and therapeutic area.
Assess Risk
Engage all stakeholders to collaboratively assess each trial for risk, with version control and audit trail.
Document Risk Strategy
Document your complete Risk Control Strategy including KRIs and thresholds.
Document your complete end-to-end risk assessment in the TMF.
Hover over the toggles to discover the features.
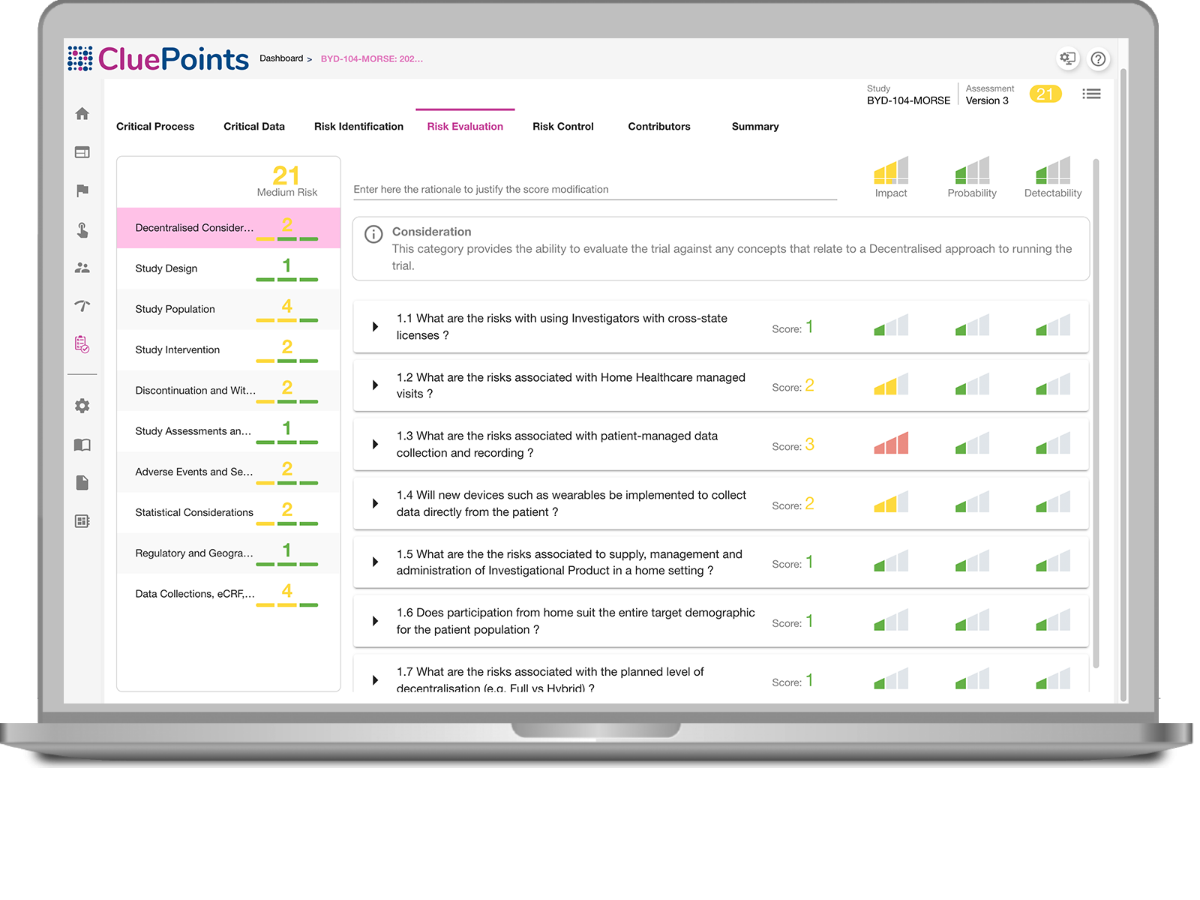
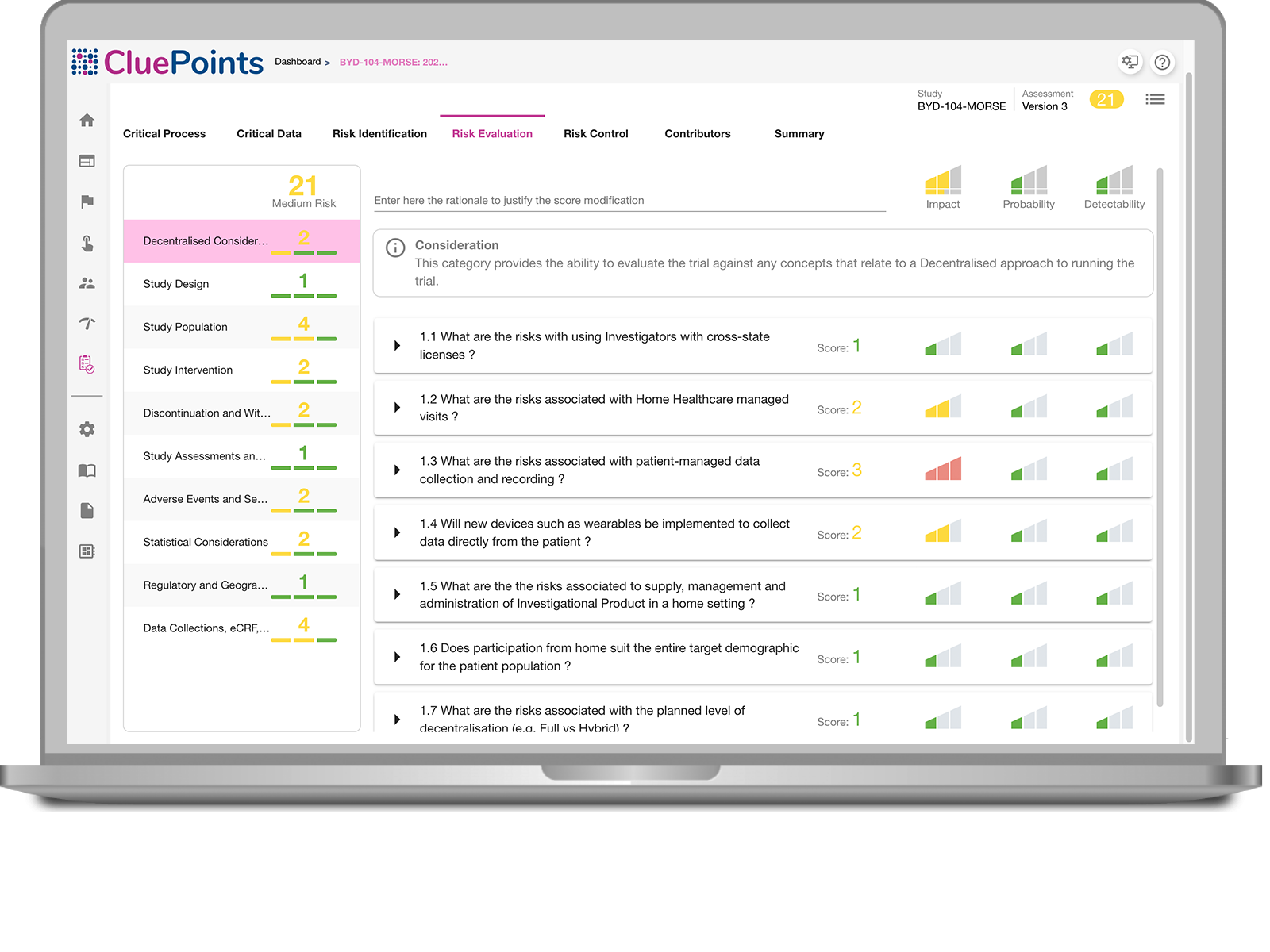
FIND STUDY RISKS FAST
Begin your ICH E6 (R2) compliance by performing a comprehensive study risk assessment that informs you as to the vulnerable areas of your trial, while providing the ability to define risk controls and monitoring capabilities so that you’re able to spot risk evolving during study execution. Maintain a dynamic, versioned risk assessment with full audit trial automatically captured from all stakeholder input.
Questionnaire
Implement Risk Assessment specific to your trial and therapeutic area.
Assess Risk
Engage all stakeholders to collaboratively assess each trial for risk, with version control and audit trail.
Document Risk Strategy
Document your complete Risk Control Strategy including KRIs and thresholds.
Document your complete end-to-end risk assessment in the TMF.
Hover over the toggles to discover the features.
- ICH E6 (R2) Compliance for performing a study risk assessment
- Define your risk control strategy, KRIs and thresholds
- Identification of critical data and processes to inform a risk assessment
- Define risk evaluation questionnaires specific to your trials/needs
- Expedite risk assessment by reusing from templates and libraries
- Support both internal and external stakeholder collaboration through the web-interface
- Create new risk assessment versions when required
- Capture all modifications with the audit trail
- Report the entire risk assessment process for filing in the TMF
- Clinical Trial Risk Assessment supporting the ICH E6 (R2) Risk-based Quality Management approach
Got a Question?
Whether you’re looking to get started or need support, we’re here to help!
Featured Resources
Tufts CSDD Impact Report
This study takes a proactive approach to understanding the dynamics between the enthusiasm around and adoption of RBQM, designed to enhance clinical trial quality and efficiency.
Featured Webinar
RBQM Adoption & ICH E6(R3) Updates
This recorded webinar dissects the Tufts CSDD Impact Report as well as new clinical trial execution models and ICH E6(R3) regulatory guidance updates.
