Patient Profiles
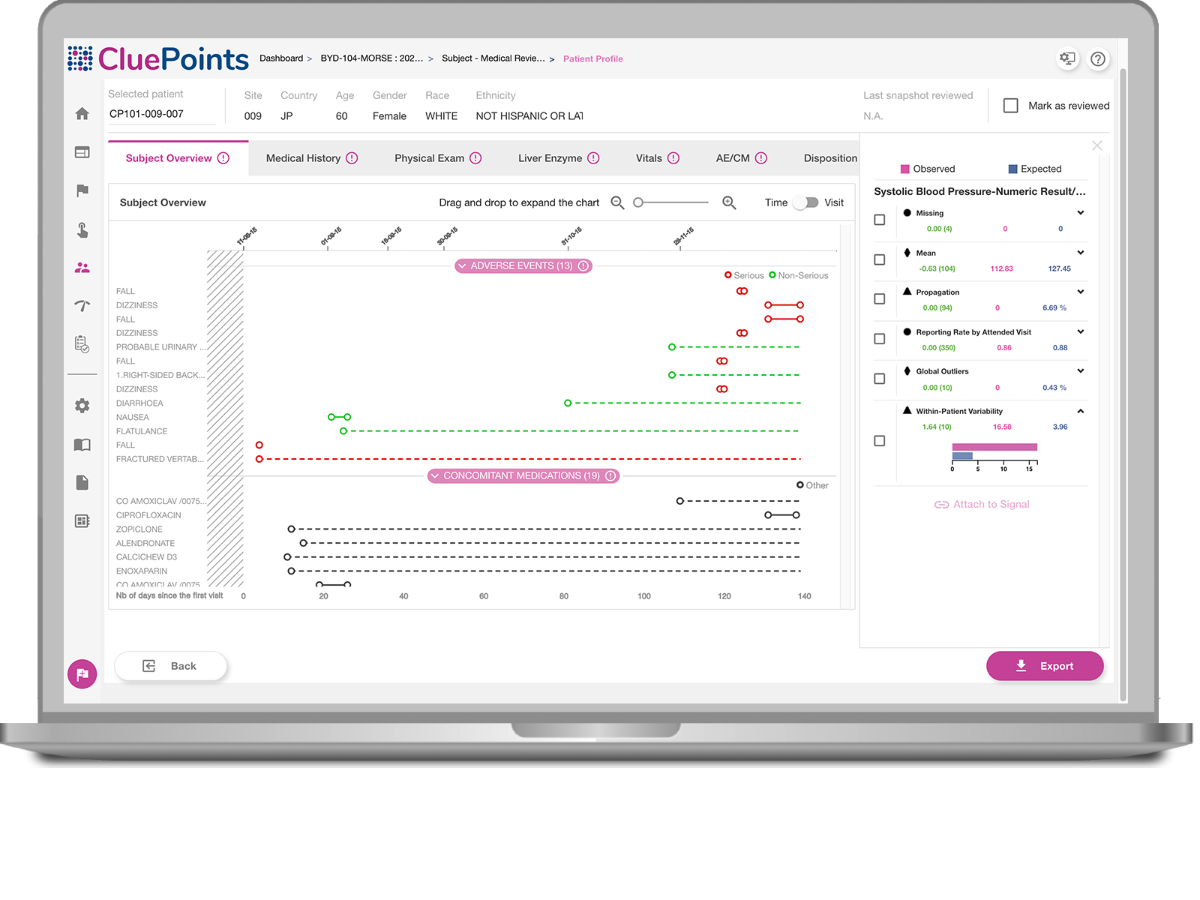
Assess Patient Data
Visualize patient data over their participation timeline.
atypical Patients
Be guided to atypical patient data by the system.
Review Progress
Record, review, and track progress.
Collaborate
Share Patient Profiles with other stakeholders.
Hover over the toggles to discover the features.
FIND ATYPICAL PATIENTS
Coupling Patient Profiles with Central Statistical Monitoring offers a more targeted approach for clinical teams to prioritize the investigation of atypical patients, by identifying anomalies in data and ranking patients by their relative degree of atypicality.
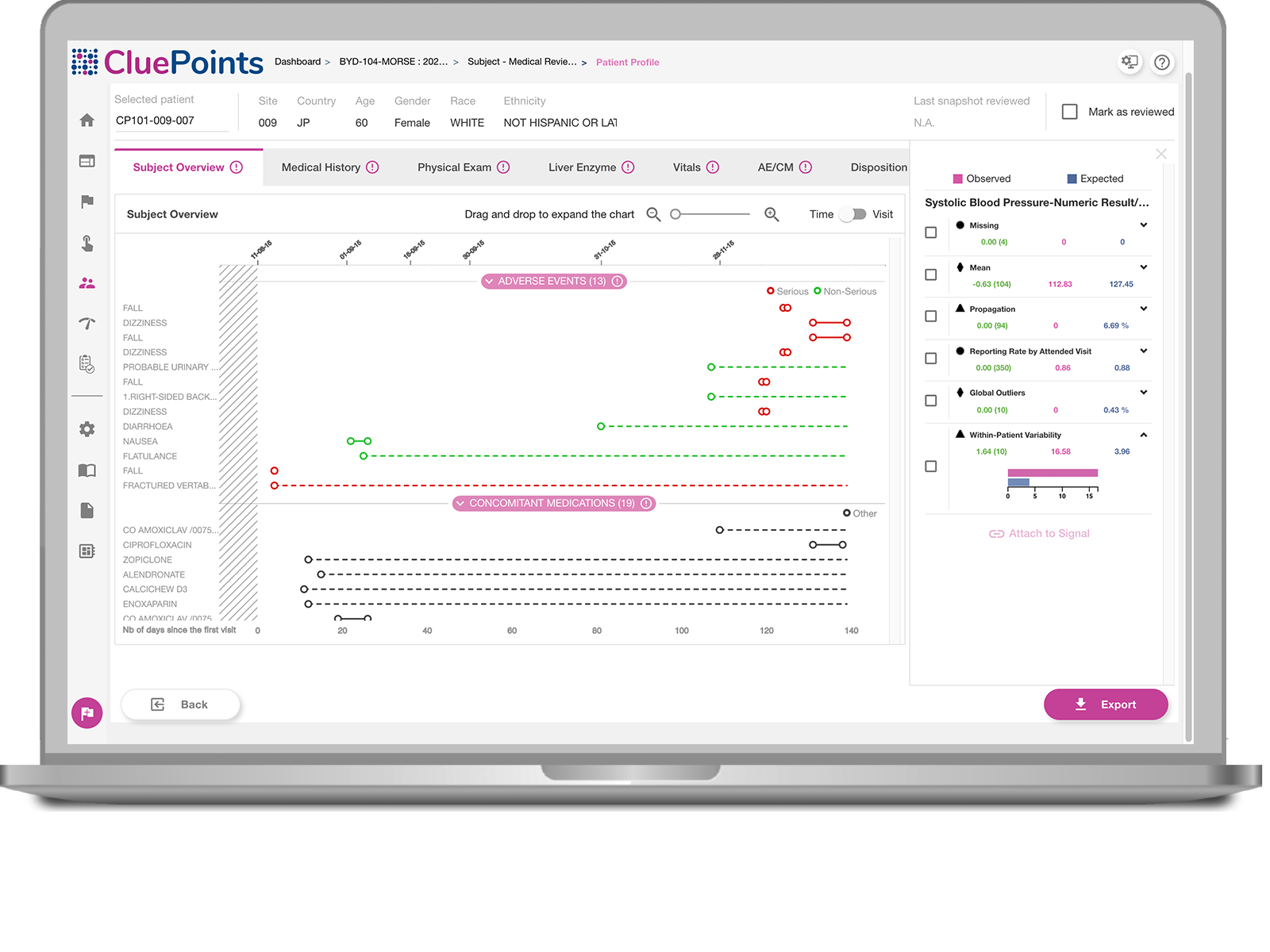
Assess Patient Data
Visualize patient data over their participation timeline.
atypical Patients
Be guided to atypical patient data by the system.
Review Progress
Record, review, and track progress.
Collaborate
Share Patient Profiles with other stakeholders.
Hover over the toggles to discover the features.
- Gain detailed insight into patient experiences.
- Target patients that matter the most.
- Benefit from a customizable set of visualization options, including the ability to visually asses a chronological view of a patient’s visit, investigational product exposure, adverse events and concomitant medications, along with any additional patient information that’s relevant.
- Quickly and effectively characterize risk signals and enable centralized medical and safety reviews.
- Perform Medical Review directly in the CluePoints platform
- Use visual Patient Profiles to explore patient data and validate operational issues
Got a Question?
Whether you’re looking to get started or need support, we’re here to help!
Featured Resources
Tufts CSDD Impact Report
This study takes a proactive approach to understanding the dynamics between the enthusiasm around and adoption of RBQM, designed to enhance clinical trial quality and efficiency.
Featured Webinar
RBQM Adoption & ICH E6(R3) Updates
This recorded webinar dissects the Tufts CSDD Impact Report as well as new clinical trial execution models and ICH E6(R3) regulatory guidance updates.
